喹啉-2-甲酰肼 | 5382-44-5
中文名称
喹啉-2-甲酰肼
中文别名
喹啉-2-卡巴肼;2-喹啉甲酸,肼;2-喹啉卡巴肼
英文名称
quinoline-2-carboxylic acid hydrazide
英文别名
quinoline-2-carbohydrazide;Chinolin-2-carbonsaeure-hydrazid;2-quinoloyl hydrazide
CAS
5382-44-5
化学式
C10H9N3O
mdl
MFCD00614750
分子量
187.201
InChiKey
JTDPJYXDDYUJBS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
溶解度:17.7 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:68
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2933499090
-
危险性防范说明:P264,P270,P301+P312,P330,P501
-
危险性描述:H302
-
储存条件:存储条件:2-8°C,密封保存,并确保环境干燥。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 喹啉-2-甲醛 quinoline 2-carbaldehyde 5470-96-2 C10H7NO 157.172 喹醛酰氯 quinolin-2-ylcarbonyl chloride 50342-01-3 C10H6ClNO 191.617 喹哪啶酸 2-quinaldic acid 93-10-7 C10H7NO2 173.171 2-甲基喹啉 2-methylquinoline 91-63-4 C10H9N 143.188 2-喹啉甲酸甲酯 quinoline-2-carboxylic acid methyl ester 19575-07-6 C11H9NO2 187.198 2-氰基喹啉 quinoline-2-carbonitrile 1436-43-7 C10H6N2 154.171 喹啉-2-羧酸乙酯 ethyl quinoline-2-carboxylate 4491-33-2 C12H11NO2 201.225 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(喹啉-2-羰基)喹啉-2-碳酰肼 N,N'-bis-(quinoline-2-carbonyl)-hydrazine 7477-47-6 C20H14N4O2 342.357 —— β-Acetyl-chinolin-2-carbonsaeurehydrazid 54570-99-9 C12H11N3O2 229.238 —— quinoline-2-carboxylic acid benzylidenehydrazide 89059-76-7 C17H13N3O 275.31 —— N-methyl-2-(quinoline-2-carbonyl)hydrazinecarbothioamide 1227872-71-0 C12H12N4OS 260.319 —— β-Benzoyl-chinolincarbonsaeure-(2)-hydrazid 59821-08-8 C17H13N3O2 291.309 —— 1-Ethyl-3-(quinoline-2-carbonylamino)thiourea 875864-10-1 C13H14N4OS 274.346 喹啉-2-羰基叠氮化物 quinoline-2-carboxylic acid azide 36802-74-1 C10H6N4O 198.184 —— N'-(4-methylbenzoyl)quinoline-2-carbohydrazide —— C18H15N3O2 305.336 —— N-[(4-chlorophenyl)methylideneamino]quinoline-2-carboxamide —— C17H12ClN3O 309.755 —— N-[(4-fluorophenyl)methylideneamino]quinoline-2-carboxamide —— C17H12FN3O 293.3 —— quinoline-2-carboxylic acid-(4-hydroxy-benzylidenehydrazide) 89059-77-8 C17H13N3O2 291.309 —— N-allyl-2-(quinoline-2-carbonyl)hydrazinecarbothioamide 1125771-71-2 C14H14N4OS 286.357 —— 4-Oxo-4-[2-(quinoline-2-carbonyl)hydrazinyl]butanoic acid 1419882-82-8 C14H13N3O4 287.275 —— N'-(4-fluorobenzoyl)quinoline-2-carbohydrazide —— C17H12FN3O2 309.3 —— 5-Oxo-5-[2-(quinoline-2-carbonyl)hydrazinyl]pentanoic acid 1419882-89-5 C15H15N3O4 301.302 —— N'-(4-chlorobenzoyl)quinoline-2-carbohydrazide —— C17H12ClN3O2 325.754 —— (Z)-4-oxo-4-[2-(quinoline-2-carbonyl)hydrazinyl]but-2-enoic acid 1419882-96-4 C14H11N3O4 285.259 —— N'-(4-bromobenzoyl)quinoline-2-carbohydrazide —— C17H12BrN3O2 370.2 —— N-[(4-methoxyphenyl)methylideneamino]quinoline-2-carboxamide —— C18H15N3O2 305.336 —— N-benzyl-2-(quinolin-2-ylcarbonyl)hydrazincarbothioamide 903178-41-6 C18H16N4OS 336.417 —— Methyl 5-oxo-5-[2-(quinoline-2-carbonyl)hydrazinyl]pentanoate 1419884-27-7 C16H17N3O4 315.329 —— N-[[4-(trifluoromethyl)phenyl]methylideneamino]quinoline-2-carboxamide —— C18H12F3N3O 343.308 —— Methyl 4-oxo-4-[2-(quinoline-2-carbonyl)hydrazinyl]butanoate 1419884-20-0 C15H15N3O4 301.302 —— salicylidene-2-quinoloylhydrazine 71658-29-2 C17H13N3O2 291.309 —— salicylidene-2-quinoloylhydrazine 71658-29-2 C17H13N3O2 291.309 —— quinoline-2-carboxylic acid-(4-dimethylamino-benzylidenehydrazide) 89059-79-0 C19H18N4O 318.378 —— N-[(2-chlorophenyl)methylideneamino]quinoline-2-carboxamide —— C17H12ClN3O 309.755 —— 1-Phenyl-3-(quinoline-2-carbonylamino)thiourea 91872-12-7 C17H14N4OS 322.39 —— N-[(2,4-dihydroxyphenyl)methylideneamino]quinoline-2-carboxamide —— C17H13N3O3 307.309 —— N'-(furan-2-carbonyl)quinoline-2-carbohydrazide —— C15H11N3O3 281.271 —— N'-(thiophene-2-carbonyl)quinoline-2-carbohydrazide —— C15H11N3O2S 297.337 —— quinoline-2-carboxylic acid-(4-acetylamino-benzylidenehydrazide) 101893-39-4 C19H16N4O2 332.362 —— N'-[(1E)-(2,5-dihydroxyphenyl)methylidene]quinoline-2-carbohydrazide —— C17H13N3O3 307.309 —— 1-Cyclohexyl-3-(quinoline-2-carbonylamino)thiourea 1125771-72-3 C17H20N4OS 328.438 —— N'-(2-chlorobenzoyl)quinoline-2-carbohydrazide —— C17H12ClN3O2 325.754 —— N'-(2-bromobenzoyl)quinoline-2-carbohydrazide —— C17H12BrN3O2 370.205 —— N'-(2-fluorobenzoyl)quinoline-2-carbohydrazide —— C17H12FN3O2 309.3 —— N-(4-chlorophenyl)-2-(quinoline-2-carbonyl)hydrazinecarbothioamide 1227872-72-1 C17H13ClN4OS 356.835 —— N-[[2-(trifluoromethyl)phenyl]methylideneamino]quinoline-2-carboxamide —— C18H12F3N3O 343.308 —— Quinoline-2-carboxylic acid [1-(3,4,5-trimethoxy-phenyl)-meth-(E)-ylidene]-hydrazide —— C20H19N3O4 365.389 —— quinoline-2-carboxylic acid piperonylidenehydrazide 101605-82-7 C18H13N3O3 319.32 —— quinoline-2-carboxylic acid-(4-chloro-3-nitro-benzylidenehydrazide) 101439-37-6 C17H11ClN4O3 354.752 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:喹啉-2-甲酰肼 在 potassium hydroxide 作用下, 以 乙醇 为溶剂, 反应 24.0h, 生成 3-[(3-Chloroanilino)methyl]-5-quinolin-2-yl-1,3,4-oxadiazole-2-thione参考文献:名称:Synthesis, molecular modeling and biological evaluation of 2-aminomethyl-5-(quinolin-2-yl)-1,3,4-oxadiazole-2(3H)-thione quinolone derivatives as novel anticancer agent摘要:A series of quinoline derivatives (4a-4o) have been synthesized and their biological activities were also evaluated as potential telomerase inhibitors. Bioassay tests demonstrated that most of the compounds exhibited substantial broad-spectrum antitumor activity against the three cancer cell lines (HepG2, SGC-7901 and MCF-7). Moreover, all the title compounds were assayed for telomerase inhibition using the TRAP-PCR-ELISA assay. Compounds 4d and 4i displayed the most potent anticancer activities, which were comparable to the positive control. Docking simulation was performed to position compounds 4d and 4i into the telomerase structure active site to determine the probable binding model. Compounds 4d and 4i with potent inhibitory activity in tumor growth inhibition may be potential anticancer agents. (C) 2012 Elsevier Masson SAS. All rights reserved.DOI:10.1016/j.ejmech.2012.11.039
-
作为产物:参考文献:名称:基于喹啉席夫氏碱及其细胞成像的高选择性和高灵敏度的铝(III)开启探针。摘要:设计,合成和评估了基于喹啉衍生物的Al3 +(3,5-二氯-2-羟基亚苄基)喹啉-2-碳酰肼(QC)的可逆Schiff碱性荧光探针。通过与Al3 +形成1:1的络合物,QC对EtOH-H2O(v / v = 1:9,pH = 6)中的Al3 +具有高灵敏度和选择性,QC对Al3 +的检出限低至0.012μM 。此外,这些结果表明,QCAl3 +的结合被F-破坏,因此该系统将来可用于监测F-。QC的增强荧光可能归因于对PET和ESIPT的抑制以及Al3 +诱导的CHEF进程的紧急性。更重要的是,质控不仅成功地用于自来水和人血清中微量Al3 +的测定,DOI:10.1016/j.saa.2017.09.007
文献信息
-
Stereoselective, solvent free, highly efficient synthesis of aldo- and keto-N-acylhydrazones applying grindstone chemistry作者:José Maurício dos Santos Filho、Savio Moita PinheiroDOI:10.1039/c7gc00730b日期:——A mild and efficient synthesis of N-acylhydrazones has been developed, applying a simple grindstone procedure, leading to a library of 51 examples exhibiting a broad variety of structural features, 21 of them described for the first time in this paper. This methodology works without any organic solvent under the catalysis of acetic acid at room temperature, promotes the formation of essentially pure已经开发出了温和而有效的N-酰基hydr酮合成方法,该方法采用了简单的磨石程序,从而产生了51个实例,这些实例均显示出多种结构特征,其中21个实例首次在本文中进行了描述。该方法在室温下在乙酸催化下无需任何有机溶剂即可工作,可促进形成基本纯净的粗产物,并避免繁琐的后处理和有害溶剂的使用以及能耗。另外,如NMR分析所支持的,其导致N-酰基hydr的立体选择性形成。
-
Dual-action inhibitors of HIF prolyl hydroxylases that induce binding of a second iron ion作者:Kar Kheng Yeoh、Mun Chiang Chan、Armin Thalhammer、Marina Demetriades、Rasheduzzaman Chowdhury、Ya-Min Tian、Ineke Stolze、Luke A. McNeill、Myung Kyu Lee、Esther C. Y. Woon、Mukram M. Mackeen、Akane Kawamura、Peter J. Ratcliffe、Jasmin Mecinović、Christopher J. SchofieldDOI:10.1039/c2ob26648b日期:——Inhibition of the hypoxia-inducible factor (HIF) prolyl hydroxylases (PHD or EGLN enzymes) is of interest for the treatment of anemia and ischemia-related diseases. Most PHD inhibitors work by binding to the single ferrous ion and competing with 2-oxoglutarate (2OG) co-substrate for binding at the PHD active site. Non-specific iron chelators also inhibit the PHDs, both in vitro and in cells. We report the identification of dual action PHD inhibitors, which bind to the active site iron and also induce the binding of a second iron ion at the active site. Following analysis of small-molecule iron complexes and application of non-denaturing protein mass spectrometry to assess PHD2·iron·inhibitor stoichiometry, selected diacylhydrazines were identified as PHD2 inhibitors that induce the binding of a second iron ion. Some compounds were shown to inhibit the HIF hydroxylases in human hepatoma and renal carcinoma cell lines.
-
4-Alkyl-1,2,4-triazole-3-thione analogues as metallo-β-lactamase inhibitors作者:Laurent Gavara、Alice Legru、Federica Verdirosa、Laurent Sevaille、Lionel Nauton、Giuseppina Corsica、Paola Sandra Mercuri、Filomena Sannio、Georges Feller、Rémi Coulon、Filomena De Luca、Giulia Cerboni、Silvia Tanfoni、Giulia Chelini、Moreno Galleni、Jean-Denis Docquier、Jean-François HernandezDOI:10.1016/j.bioorg.2021.105024日期:2021.8(BLs), including the highly worrying carbapenemases. Whereas inhibitors of these enzymes were recently marketed, they only target serine-carbapenemases (e.g. KPC-type), and no clinically useful inhibitor is available yet to neutralize the class of metallo-β-lactamases (MBLs). We are developing compounds based on the 1,2,4-triazole-3-thione scaffold, which binds to the di-zinc catalytic site of MBLs在革兰氏阴性菌中,对 β-内酰胺类抗生素产生耐药性的主要机制是产生一种或多种 β-内酰胺酶 (BLs),包括令人担忧的碳青霉烯酶。尽管这些酶的抑制剂最近上市,但它们仅针对丝氨酸-碳青霉烯酶(例如 KPC 型),尚无临床上有用的抑制剂来中和金属-β-内酰胺酶 (MBL) 类。我们正在开发基于 1,2,4-三唑-3-硫酮支架的化合物,该支架以原始方式与 MBL 的二锌催化位点结合,我们之前报道了其产生广谱抑制剂的潜力。然而,到目前为止,在微生物测定中只能观察到适度的抗生素增强作用,需要进一步探索以改善外膜渗透。这里,我们合成并表征了一系列在杂环的 4 位具有不同官能化烷基链的化合物。我们发现在烷基链末端存在羧基会产生有效的 VIM 型酶抑制剂K i值在 μM 到亚 μM 范围内,并且该烷基链必须更长或等于丙基链。该结果证实了羧基官能团对 1,2,4-三唑-3-硫酮杂环的 4-取代基的重要性。如
-
Synthesis of Dihydrothienopyridine Derivatives Fused with Triazole Rings作者:Péter Ábrányi-Balogh、Mátyás Milen、András Dancsó、Dávid Frigyes、László Pongó、György KeglevichDOI:10.1002/hc.21087日期:2013.5New dihydro[3,2-c][1,2,4]triazolo[4,3-a]pyridines were synthesized by the reaction of 4-(methylsulfanyl)-6,7-dihydrothieno[3,2-c]pyridine with acid hydrazides. One bis(dihydrothienotriazolo-pyridine) was also prepared. In a few cases, the corresponding intermediate could be detected by LC-MS. The bromophenyl derivative was involved in Suzuki and Sonogashira cross-coupling reactions. © 2013 Wiley Periodicals
-
Design and Discovery of Novel Antifungal Quinoline Derivatives with Acylhydrazide as a Promising Pharmacophore作者:Yu-Dong Yang、Ying-Hui He、Kun-Yuan Ma、Hu Li、Zhi-Jun Zhang、Yu Sun、Yu-Ling Wang、Guan-Fang Hu、Ren-Xuan Wang、Ying-Qian LiuDOI:10.1021/acs.jafc.1c00670日期:2021.8.4were synthesized and evaluated for their fungicidal activity. Most of these compounds exhibited excellent fungicidal activity in vitro. Significantly, compound 2e displayed the superior in vitro antifungal activity against Sclerotinia sclerotiorum, Rhizoctonia solani, Botrytis cinerea, and Fusarium graminearum with the EC50 values of 0.39, 0.46, 0.19, and 0.18 μg/mL, respectively, and were more potent受天然 2-喹啉羧酸衍生物的启发,合成了一系列含有酰肼、酰腙、磺酰肼、恶二唑、噻二唑或三唑部分的喹啉化合物,并评估了它们的杀菌活性。大多数这些化合物在体外表现出极好的杀真菌活性。值得注意的是,化合物2e对核盘菌、立枯丝核菌、灰葡萄孢和禾谷镰刀菌显示出优异的体外抗真菌活性,EC 50值分别为 0.39、0.46、0.19 和 0.18 μg/mL,并且更有效。多菌灵(EC50,0.68,0.14,> 100,和0.65微克/毫升,分别地)。此外,化合物2e可以抑制禾谷镰刀菌的孢子萌发。初步机理研究表明,化合物2e可引起细胞壁和液泡形态异常、线粒体丢失、膜通透性增加和细胞内容物释放。这些结果表明,化合物2e表现出优异的杀真菌活性,可能是对抗植物真菌病害的潜在杀真菌候选物。
表征谱图
-
氢谱1HNMR
-
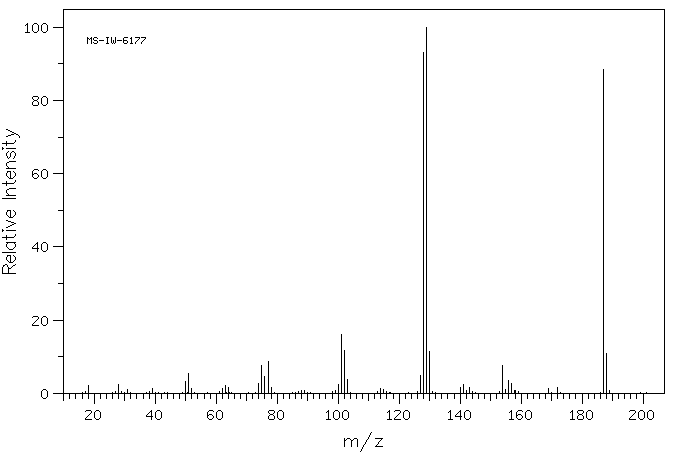
质谱MS
-
碳谱13CNMR
-
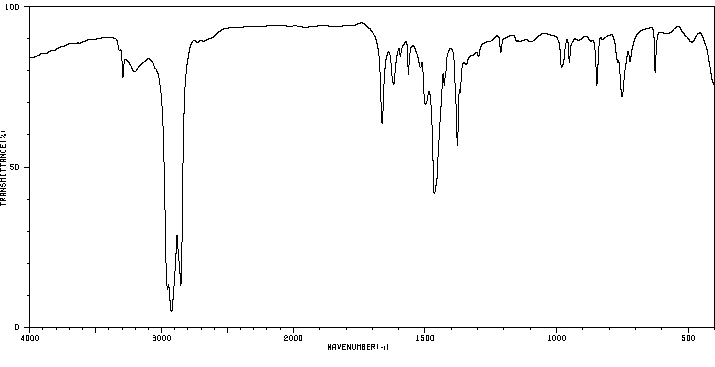
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43