对薄荷-1(7),3-二烯 | 99-84-3
中文名称
对薄荷-1(7),3-二烯
中文别名
——
英文名称
beta-terpinene
英文别名
4-methylene-1-(1-methylethyl)cyclohexene;β-terpinene;β-terpinen;terpinene;p-mentha-1(7),3-diene;p-Mentha-1(7),3-dien;4-methylidene-1-propan-2-ylcyclohexene
CAS
99-84-3
化学式
C10H16
mdl
——
分子量
136.237
InChiKey
SCWPFSIZUZUCCE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:185.55°C (rough estimate)
-
密度:0.8380
-
LogP:4.147 (est)
-
保留指数:1049;1051;1019
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:描述:对薄荷-1(7),3-二烯 在 disodium hydrogenphosphate 、 双氧水 、 六氟丙酮 作用下, 以 various solvent(s) 为溶剂, 反应 6.0h, 以97%的产率得到参考文献:名称:Hexafluoro-2-propanol 中的 Hexafluoroacetone:一种用于过氧化氢水溶液环氧化的高活性介质摘要:将六氟-2-丙醇和催化剂量的六氟丙酮结合在一起,可提供一种多功能介质,用于多种烯烃与过氧化氢水溶液的环氧化反应。DOI:10.1055/s-2001-16037
-
作为产物:描述:(4-isopropyl-cyclohex-3-enyliden)-acetic acid 生成 对薄荷-1(7),3-二烯参考文献:名称:Wallach, Justus Liebigs Annalen der Chemie, 1907, vol. 357, p. 72摘要:DOI:
文献信息
-
Conjugated Dienes as Prohaptens in Contact Allergy: In Vivo and in Vitro Studies of Structure−Activity Relationships, Sensitizing Capacity, and Metabolic Activation作者:Moa Andresen Bergström、Kristina Luthman、J. Lars G. Nilsson、Ann-Therese KarlbergDOI:10.1021/tx060006n日期:2006.6.1alpha-terpinene were demonstrated to be prohaptens able to induce contact allergy. The difference in sensitizing capacity of conjugated dienes as compared to alkenes with isolated double bonds was found to be due to the high reactivity and sensitizing capacity of the allylic epoxides metabolically formed from conjugated dienes. We recommend that these structure-activity relationship rules are incorporated into在开发用于预测接触变应原活性的体外/计算机方法中,引起了极大的兴趣。然而,许多提出的方法没有考虑到通过皮肤代谢使赋形剂对敏化剂的活化。结果,可以销售含有有效敏化剂的消费品。为了识别proepttens,必须对其结构-活性关系及其激活机制进行研究。在目前的研究中,我们研究了烯烃前体的构效关系。设计了一系列七个相同结构的烯烃(1-7),但双键的数量和位置有所变化,并使用鼠类局部淋巴结试验筛选了它们的敏化能力。在谷胱甘肽存在下,还将化合物1-7与肝微粒体一起温育以捕获并鉴定反应性代谢物。还研究了三种烯烃(9-11)到环氧化物(12-15)的代谢转化以及它们的敏化能力的比较。我们的结果表明,在六元环中或与六元环结合的共轭二烯是可能被代谢活化为环氧化物并与GSH结合的肽。含有孤立的双键的相关烯烃和无环共轭二烯显示为弱或非敏化剂。首次证明天然存在的单萜类α-水芹烯,β-水芹烯和α-萜品烯能够诱导接触过
-
Catalytic activity of the VIII Group metals in the hydrogenation and isomerization of α- and β-pinenes作者:I. V. Deliy、I. L. SimakovaDOI:10.1007/s11172-008-0279-1日期:2008.10contains the products of double bond hydrogenation, viz., cis- and trans-pinanes. The Ru, Rh, and Pd metals have a higher catalytic activity in β-pinene isomerization than Ir and Pt. Among the VIII Group metals studied, the Pd-based catalyst has the highest catalytic activity in double bond isomerization of α- and β-pinenes. The general scheme of the mechanism of hydrogenation and isomerization of α- and β-pinenes
-
Wallach, Justus Liebigs Annalen der Chemie, 1908, vol. 362, p. 287作者:WallachDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
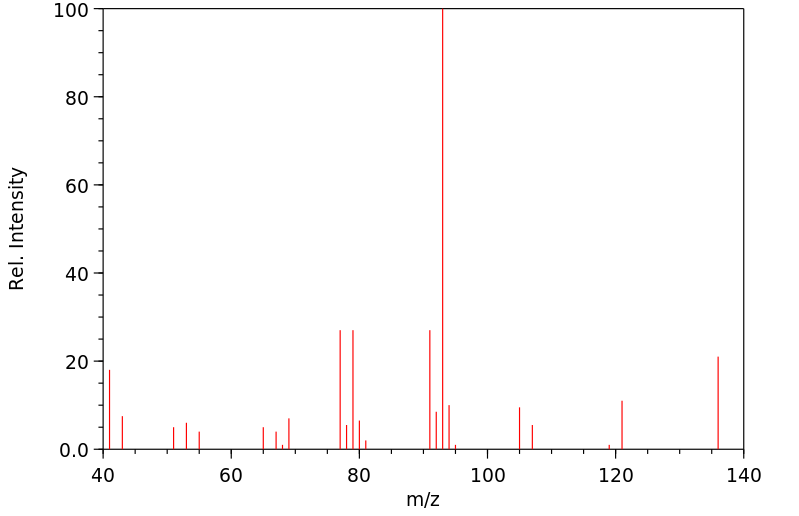
质谱MS
-
碳谱13CNMR
-
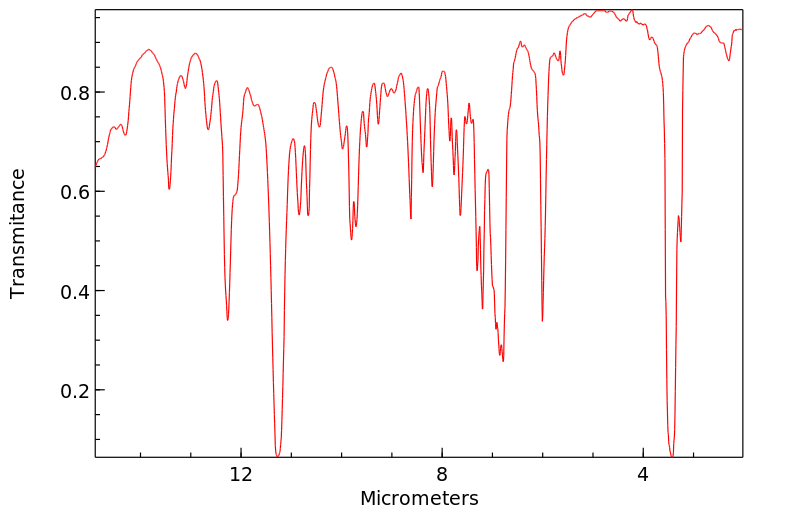
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸