(Z)-5,5-二甲基己-2-烯 | 39761-61-0
中文名称
(Z)-5,5-二甲基己-2-烯
中文别名
(Z)-5,5-二甲基-2-己烯
英文名称
cis-5,5-Dimethyl-hexen-(2)
英文别名
2-Hexene, 5,5-dimethyl-, (Z)-;(Z)-5,5-dimethylhex-2-ene
CAS
39761-61-0
化学式
C8H16
mdl
——
分子量
112.215
InChiKey
NWZJLSKAFZXSQH-WAYWQWQTSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-103.01°C (estimate)
-
沸点:120.2°C (estimate)
-
密度:0.7125
-
保留指数:732.5;710.2;709
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 反式-2,2-二甲基-3-己烯 (E)-2,2-dimethyl-3-hexene 690-93-7 C8H16 112.215
反应信息
-
作为反应物:描述:4-苯基-1,2,4-三唑啉-3,5-二酮 、 (Z)-5,5-二甲基己-2-烯 以 二氯甲烷 为溶剂, 生成 4-Phenyl-1-((E)-1,4,4-trimethyl-pent-2-enyl)-[1,2,4]triazolidine-3,5-dione参考文献:名称:三唑啉二酮与顺式烯烃的反应。区域选择性高的烯反应摘要:三唑啉二酮与不对称的顺式烯烃的烯反应是区域特异性的,并且在双键较大的烷基上显示出烯丙基氢的优先提取。DOI:10.1016/s0040-4039(01)93384-2
-
作为产物:描述:2,2-二甲基-5-己烯-1-醇 在 吡啶 、 碘 、 lithium pyrrolidide 、 三苯基膦 作用下, 以 四氢呋喃 、 苯 为溶剂, 反应 24.0h, 生成 (Z)-5,5-二甲基己-2-烯参考文献:名称:Competing radical, carbanion, and carbene pathways in the reactions of hindered primary alkyl halides with lithium dialkylamides摘要:A variety of methods were utilized to study the mechanism of reaction of 6-iodo-5,5-dimethyl-1-hexene and its bromo, chloro, and tosylate derivatives with LDA and several other lithium dialkylamides. In the reaction of 6-iodo-5,5-dimethyl-1-hexene with LDA in THF, radical, carbanion, and carbene pathways occurred simultaneously. However, when the corresponding bromide was allowed to react with LDA, the radical pathway was minor and when the corresponding chloride or tosylate was allowed to react with LDA, no evidence for radical products was observed. This is the first time that competing radical, carbanion, and carbene pathways have been detected in the reaction of a primary alkyl halide with any nucleophile.DOI:10.1021/jo00054a028
文献信息
-
An easy synthesis of the 2-phenylsulfonyl-substituted allylic bromides and acetates and their reactivity towards nucleophiles作者:P. Auvray、P. Knochel、J.F. NormantDOI:10.1016/s0040-4039(00)85142-4日期:1986.1The reaction of phenyl vinyl sulfone with various aldehydes in the presence of a catalytic amount of DABCO furnishes in good yields teh corresponding 2-phenylsulfonyl-substituted alcohols which can be easily converted into their acetates or into their allylically rearranged bromides . These reagents, in turn, react with nucleophides (ketone enolates and cuprates) with an allylic rearrangement (SN2′
-
Reactions of triazolinedione with alkenes. A remarkable geminal selectivity.作者:Michael Orfanopoulos、Yiannis Elemes、Manolis StratakisDOI:10.1016/s0040-4039(00)97956-5日期:1990.1The ene reaction of N-Phenyl-1,2,4-triazoline-3,5-dione with alkenes shows a remarkable preference for hydrogen abstraction from the group which is geminal to the larger substituent of the double bond. These results require that the dominant effect in the transition state of the ene reaction is the nonbonded interactions.N-苯基-1,2,4-三唑啉-3,5-二酮与烯烃的烯键反应显示出明显的偏爱从双键的较大取代基团中提取氢。这些结果要求烯反应过渡态的主要作用是非键相互作用。
-
Regioselective reaction of singlet oxygen with cis-alkenes作者:Michael Orfanopoulos、Manolis Stratakis、Yiannis ElemesDOI:10.1016/s0040-4039(01)80532-3日期:1989.1The reaction of singlet oxygen with cis olefins is regioselective and shows a general preference for hydrogen abstraction on the larger alkyl group of the double bond.单线态氧与顺式烯烃的反应是区域选择性的,并且在双键的较大烷基上显示出一般优先选择氢。
-
Factors Influencing the Direction of Elimination in Ester Pyrolyses作者:Robert A. Benkeser、James J. Hazdra、Merwyn L. BurrousDOI:10.1021/ja01529a031日期:1959.10
-
The Reaction of Mesityl Oxide with t-Butylmagnesium Chloride作者:Frank C. Whitmore、D. P. J. Goldsmith、N. C. Cook、J. C. Yarze、G. G. EckeDOI:10.1021/ja01157a016日期:1950.1
表征谱图
-
氢谱1HNMR
-
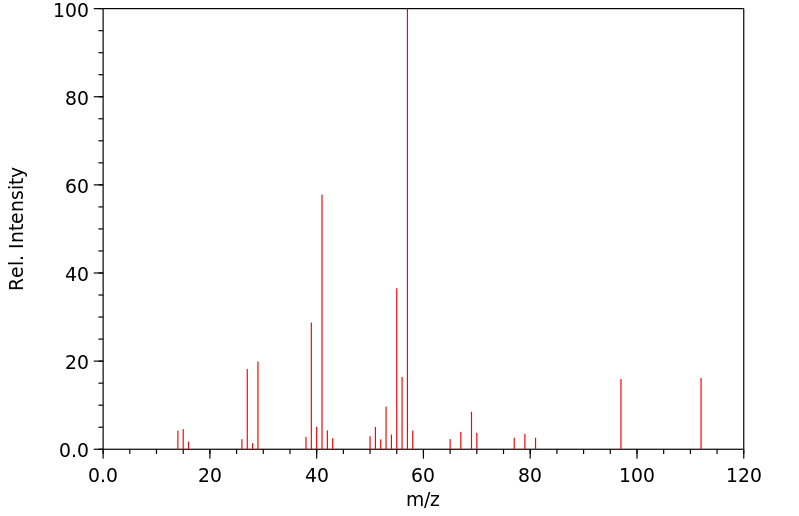
质谱MS
-
碳谱13CNMR
-
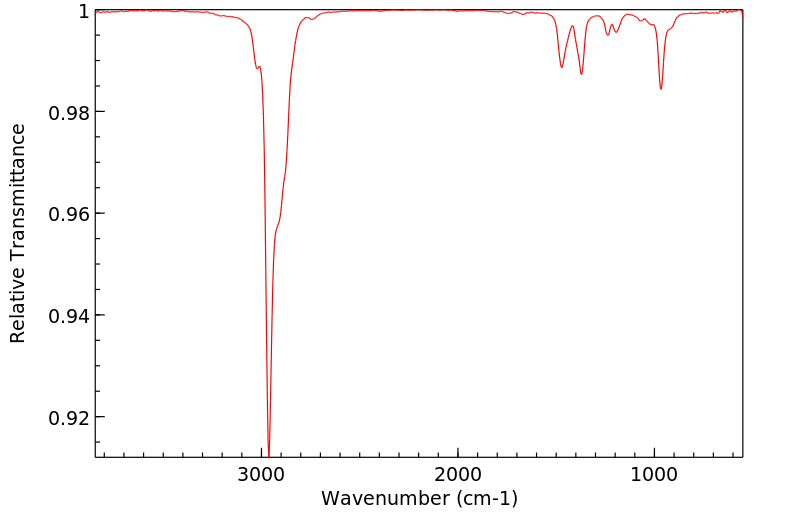
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-