1,1'-联(环辛基) | 6708-17-4
中文名称
1,1'-联(环辛基)
中文别名
——
英文名称
bicyclooctyl
英文别名
1,1'-bicyclooctyl;bicyclo-octane;cyclooctylcyclooctane
CAS
6708-17-4
化学式
C16H30
mdl
——
分子量
222.414
InChiKey
NLUNLVTVUDIHFE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:10-11 °C
-
沸点:100 °C(Press: 1 Torr)
-
密度:0.863±0.06 g/cm3(Predicted)
-
保留指数:1808
计算性质
-
辛醇/水分配系数(LogP):7.9
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:描述:6,9-Methylene-11,15-dihydroxy-17-methyl-20-isopropylideneprost-5,13-dienoic acid 、 、 1,1'-联(环辛基) 生成 6,9-Methylene-11,15-dihydroxy-20-isopropylideneprost-5,13-dienoic acid参考文献:名称:Composition for forming antithrombotic medical appliances摘要:一种组合物,包括一种聚合物,其中分散着一种前列环素衍生物,所述前列环素衍生物从以下化合物组中选择:(I)式化合物,其中R1代表氢或低碳基,R2代表C3-C12烷基或C3-C12烯基,n为1至5的整数;以及其药学上可接受的盐,可以用来制造医疗器具,从中前列腺素具有抗血栓作用,将逐渐和受控地释放。公开号:US05153003A1
-
作为产物:描述:参考文献:名称:CuI-酰胺双(膦)通过 C(sp3)–H 活化催化未活化烷烃的 C(sp3)–C(sp3) 直接同源和异源偶联摘要:在此,我们提出了一种 Cu I-二聚体,[Cu I {Ph 2 PC 6 H 4 C( O )NC 6 H 4 PPh 2 - o }] 2 ,它催化了苄基和具有优异产率和区域选择性的环烷烃衍生物。该方法非常简单并且可以容忍各种功能。在 Cu-N 键断裂和氮质子化中观察到协同金属-配体协同作用,并促进双功能途径,最大限度地减少催化中间体的自由能波纹。DOI:10.1039/d4cc01119h
文献信息
-
Zur Kenntnis des Kohlenstoffringes. 58. Mitteilung Über die Reaktion einiger vielgliedriger Cycloalkyl-bromide mit Magnesium作者:L. Ruzicka、P. Barman、V. PrelogDOI:10.1002/hlca.19510340144日期:——10gliedrigen Cycloalkyl-bromide in ätherischer Lösung mit Magnesium und Umsetzung des erhaltenen Reaktionsproduktes mit Kohlendioxyd wurden neben kleineren Mengen Cycloalkan-carbonsäuren hauptsächlich Kohlenwasserstoff-Gemische erhalten, die aus Cycloalken, Cycloalkan und Dicycloalkyl bestehen. Dieses ungewöhnliche Verhalten wird auf die leichte Bildung der freien Cycloalkyl-Radikale mit einer mittleren Ringgliederzahl
-
Alkane functionalization on a preparative scale by mercury-photosensitized cross-dehydrodimerization作者:Stephen H. Brown、Robert H. CrabtreeDOI:10.1021/ja00190a032日期:1989.45-trioxacyclohexane < ethanol < isobutane < THF < Etsub 3}SiH. The observed selectivities generally parallel those for homodimerization, reported in the preceding paper, but certain differences are noted, and reasons for the differences are proposed. The bond-dissociation energymore » of Etsub 3}SiH is estimated from the reactivity data to be 90 kcal/mol. Eleven new carbinols are synthesized. 39 refs., 6 tabs烷烃可以通过烷烃与醇、醚或硅烷之间的汞光敏反应以高转化率和高化学和量子产率在多克规模下官能化,得到同二聚体和交叉脱氢二聚体。由于同二聚体和交叉二聚体的极性差异很大,因此产物混合物的分离通常特别容易。当通过改变液体组成来调节气相中组分的比例时,也可以偏向产物组成。这对于最大化化学产率或通过有利于形成最容易分离的化合物对来简化分离是有用的。讨论了反应的机理基础,并详细描述了许多特定类型的合成,例如 2,2-二取代的甲醇。交叉二聚的选择性被证明超过了均二聚的选择性,并讨论了原因。不同化合物和化合物类别的相对反应性为 MeOH < 对二恶烷 < 环己烷 < 1,3,5-三恶六环己烷 < 乙醇 < 异丁烷 < THF < Etsub 3}SiH。观察到的选择性通常与前一篇论文中报道的均二聚化的选择性相似,但注意到了某些差异,并提出了差异的原因。Etsub 3}SiH 的键离解能根据反应性数据估计为
-
Reactions of hydrogen atoms produced by microwave discharge with olefins in acetone and toluene作者:Ahuva. Beeri、Elisha. Berman、Rosita. Vishkautsan、Yehuda. MazurDOI:10.1021/ja00280a062日期:1986.10photolysis of thiols and tert-butyl peroxyformate. In the gas phase, reactions of H atoms generally result in the vibrationally excited radicals which lead to extensive fragmentations. However, in the liquid phase, the atoms were found to be less reactive. The reactions of H atoms with olefins were performed in a flow system at 2 torr, the H atoms being generated by microwave discharge (2540-MHz, 60-W output)该实验室以前的结果表明,微波放电是一种方便且有效的氧原子源,用于有机合成,处于凝聚相。现在作者报告说,这个来源也提供了一种产生相同目的的氢原子的极好方法。H 原子与有机底物在液相和气相中的反应已被广泛研究。为此目的,主要通过 H2 的放电、水和有机液体的辐射分解以及硫醇和过甲酸叔丁酯的光解产生 H 原子。在气相中,H 原子的反应通常会产生振动激发的自由基,从而导致广泛的碎裂。然而,在液相中,发现原子的反应性较低。H 原子与烯烃的反应在 2 torr 的流动系统中进行,由 H2 和 He (1:50) 的混合物的微波放电(2540-MHz,60-W 输出)产生的 H 原子。排出的气体通过纯液体或基材溶液。由于发现丙酮对 H 原子呈惰性,因此作者在这些反应中将其用作溶剂。
-
A Structure–Activity Study of Ni-Catalyzed Alkyl–Alkyl Kumada Coupling. Improved Catalysts for Coupling of Secondary Alkyl Halides作者:Peng Ren、Oleg Vechorkin、Kim von Allmen、Rosario Scopelliti、Xile HuDOI:10.1021/ja200270k日期:2011.5.11of several well-defined Ni catalysts that are significantly more active and efficient than the pincer complex [((Me)N(2)N)NiCl] for the coupling of secondary alkyl halides. The best two catalysts are [((H)NN)Ni(PPh(3))Cl] and [((H)NN)Ni(2,4-lutidine)Cl]. The improved activity and efficiency was attributed to the fact that phosphine and lutidine ligands in these complexes can dissociate from the Ni对 Ni 催化的烷基-烷基熊田型交叉偶联反应进行了结构-活性研究。合成了一系列新的镍(II)配合物,包括具有三齿钳状双(氨基)酰胺配体((R)N(2)N)和具有双齿混合氨基-酰胺配体((R)NN)的配合物并对其进行了结构表征. 这些配合物的配位几何形状从方形平面、四面体到方形金字塔形。该配合物已被研究作为未活化的烷基卤化物,特别是仲烷基碘化物与烷基格氏试剂交叉偶联的预催化剂。与先前报道的 Ni 钳形复合物 [((Me)N(2)N)NiCl] 获得的结果进行了比较。预催化剂中的金属转移位点对于催化是必需的。预催化剂的配位几何形状和自旋状态影响很小或没有影响。这项工作导致发现了几种明确定义的 Ni 催化剂,这些催化剂比钳形复合物 [((Me)N(2)N)NiCl] 更活跃、更有效,用于偶联仲烷基卤化物。最好的两种催化剂是 [((H)NN)Ni(PPh(3))Cl] 和 [((H)NN)Ni(2
-
Mono- and Disubstituted N,N-Dialkylcyclopropylamines from Dialkylformamides via Ligand-Exchanged Titanium–Alkene Complexes Part 79 in the series “Cyclopropyl Building Blocks for Organic Synthesis”. For part 78 see: M. Gensini, I. Kozhushkov, S. Yufit, K. Howard, M. Es-Sayed, de Meijere, Eur. J. Org. Chem. 2002, 2499–2507; part 77: H. Nüske, S. Bräse, I. Kozhushkov, M. Noltemeyer, M. Es-Sayed, de Meijere, Chem. Eur. J. 2002, 8, 2350–2369.作者:Armin de Meijere、Craig M. Williams、Alexandre Kourdioukov、Sergei V. Sviridov、Vladimir Chaplinski、Markus Kordes、Andrei I. Savchenko、Christian Stratmann、Mathias NoltemeyerDOI:10.1002/1521-3765(20020816)8:16<3789::aid-chem3789>3.0.co;2-r日期:2002.8.16Dibenzylformamide was treated with cyclohexylmagnesium bromide in the presence of either titanium tetraisopropoxide or methyltitanium triisopropoxide and a variety of cyclic and acyclic alkenes and alkadienes to give new mono- and disubstituted as well as bicyclic dialkylcyclopropylamines (Tables 1-3) in yields ranging from 18 to 90 % (in most cases around 55 %). 3-Benzyl-6-(N,N-dibenzylamino)-3-azabicyclo[3在四异丙氧基钛或甲基三异丙氧基钛和各种环和无环烯烃与链二烯的存在下,用环己基溴化镁处理二苄基甲酰胺,得到新的单取代和双取代的以及双环的二烷基环丙胺(表1-3),收率范围为18至22。 90%(大多数情况下约为55%)。3-苄基-6-(N,N-二苄氨基)-3-氮杂双环[3.1.0]己烷(10 a)和正交双保护的3-叔丁氧基羰基-6-(N,N-二苄基)-3-氮杂双环[3.1.0]己烷(10 d)以及类似的6-(N,N-二苄氨基)双环[3.1.0]己烷(12)以纯正的非对映异构体形式获得(87、90和90%)。 N-苄基吡咯啉(15a),N-Boc-吡咯啉(15d; Boc =叔丁氧羰基)和环戊烯(分别为88%)。1,3-丁二烯(52)和取代的1,3-丁二烯也被氨基环丙烷化得很好,以良好的收率(51-64%)得到2-乙烯基环丙胺。除烯基和芳基取代的化合物外,N,N-二苄基环丙胺可通过催化氢化反应脱苄基成伯环丙胺,如10
表征谱图
-
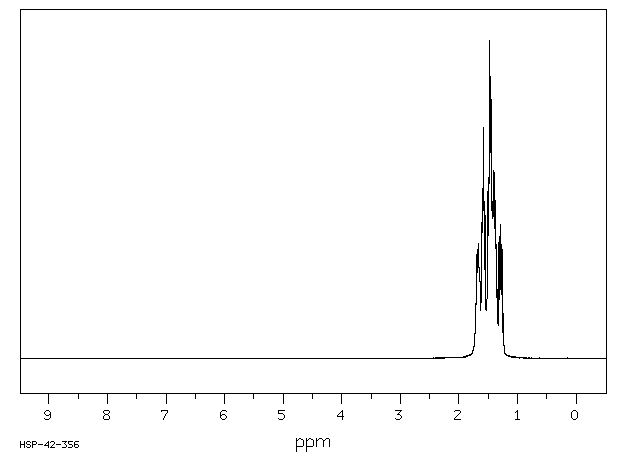
氢谱1HNMR
-
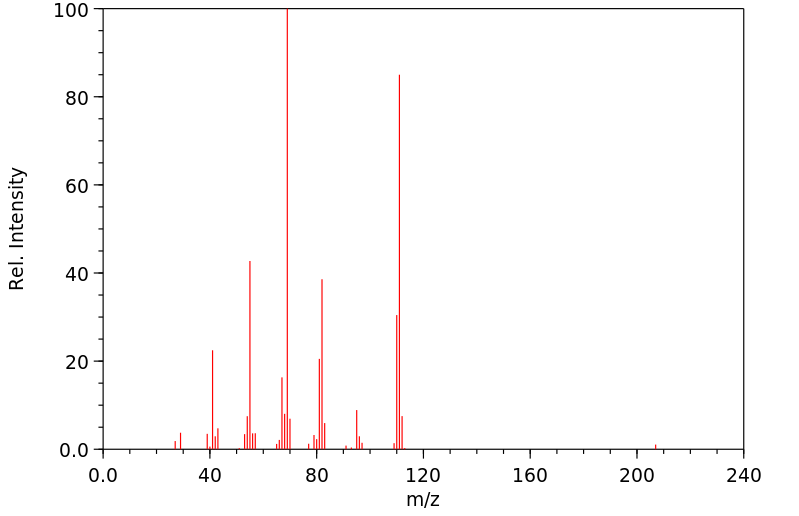
质谱MS
-
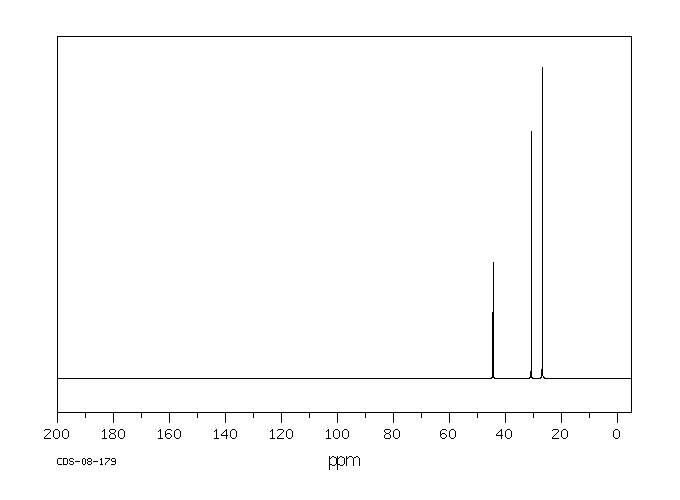
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷