1,16-二溴十六烷 | 45223-18-5
中文名称
1,16-二溴十六烷
中文别名
——
英文名称
1,16-dibromohexadecane
英文别名
——
CAS
45223-18-5
化学式
C16H32Br2
mdl
MFCD00039195
分子量
384.238
InChiKey
OTFBUFWEFKVFFR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56.2-56.7 °C
-
沸点:397.8±10.0 °C(Predicted)
-
密度:1.204±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):8.6
-
重原子数:18
-
可旋转键数:15
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903399090
-
危险性防范说明:P264,P280,P302+P352,P337+P313,P305+P351+P338,P362+P364,P332+P313
-
危险性描述:H315,H319
-
储存条件:2-8℃
SDS
制备方法与用途
用途
1,16-二溴十六烷是一种有机中间体,可通过将1,16-十六烷二醇进行溴代反应制得。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,6-二溴己烷 1 ,6-dibromohexane 629-03-8 C6H12Br2 243.969 16-溴-1-十六烷醇 16-bromohexadecan-1-ol 59101-28-9 C16H33BrO 321.341 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 16-bromo-1-hexadecanethiol 1362855-77-3 C16H33BrS 337.408 16-溴-1-十六烷醇 16-bromohexadecan-1-ol 59101-28-9 C16H33BrO 321.341
反应信息
-
作为反应物:描述:1,16-二溴十六烷 在 氢氧化钾 、 potassium ethoxide 、 potassium carbonate 作用下, 以 二甲基亚砜 为溶剂, 反应 0.67h, 生成 1-tosylazacycloheptadecane参考文献:名称:Gargano, Patrizia; Mandolini, Luigi, Gazzetta Chimica Italiana, 1982, vol. 112, # 1/2, p. 31 - 34摘要:DOI:
-
作为产物:描述:参考文献:名称:Conformationally-locked N-glycosides: Exploiting long-range non-glycone interactions in the design of pharmacological chaperones for Gaucher disease摘要:Pyranoid-type glycomimetics having a cis-1,2-fused glucopyranose-2-alkylsulfany1-1,3-oxazoline (Glc-PSO) structure exhibit an unprecedented specificity as inhibitors of mammalian beta-glucosidase. Notably, their inhibitory potency against human beta-glucocerebrosidase (GCase) was found to be strongly dependent on the nature of aglycone-type moieties attached at the sulfur atom. In the particular case of omega-substituted hexadecyl chains, an amazing influence of the terminal group was observed. A comparative study on a series of Glc-PSO derivatives suggests that hydrogen bond acceptor functionalities, e.g. fluoro or methyloxycarbonyl, significantly stabilize the Glc-PSO:GCase complex. The S-(16-fluorohexadecyl)-PSO glycomimetic turned out to be a more potent GCase competitive inhibitor than ambroxol, a non glycomimetic drug currently in pilot trials as a pharmacological chaperone for Gaucher disease. Moreover, the inhibition constant increased by one order of magnitude when shifting from neutral (pH 7) to acidic (pH 5) media, a favorable characteristic for a chaperone candidate. Indeed, the fluoro-PSO derivative also proved superior to ambroxol in mutant GCase activity enhancement assays in N370S/N370S Gaucher fibroblasts. The results presented here represent a proof of concept of the potential of exploiting long-range non-glycone interactions for the optimization of glycosidase inhibitors with chaperone activity. (C) 2014 Elsevier Masson SAS. All rights reserved.DOI:10.1016/j.ejmech.2014.11.002
文献信息
-
Nickel‐Catalyzed Inter‐ and Intramolecular Aryl Thioether Metathesis by Reversible Arylation作者:Tristan Delcaillau、Alessandro Bismuto、Zhong Lian、Bill MorandiDOI:10.1002/anie.201910436日期:2020.1.27A nickel-catalyzed aryl thioether metathesis has been developed to access high-value thioethers. 1,2-Bis(dicyclohexylphosphino)ethane (dcype) is essential to promote this highly functional-group-tolerant reaction. Furthermore, synthetically challenging macrocycles could be obtained in good yield in an unusual example of ring-closing metathesis that does not involve alkene bonds. In-depth organometallic
-
An Efficient Procedure for the 1-Alkylation of 2-Nitroimidazoles and the Synthesis of a Probe for Hypoxia in Solid Tumours作者:Anthony Long、John Parrick、Richard J. HodgkissDOI:10.1055/s-1991-26552日期:——The N-alkylation of 2-nitroimidazole through its 'naked' anion, formed from its alkali metal salts in the presence of crown ethers and in homogeneous solution, is shown to be a useful preparative procedure. The reactions of α,Ï-dihaloalkanes can be controlled to afford either the mono- or diheteroarylalkane. The procedure has been used to alkylate theophylline and to prepare a compound of use as a probe to identify, locate and quantify hypoxia in sections from solid tumours.
-
THE ELECTROLYSIS OF ω-BROMOCARBOXYLIC ACIDS作者:R.G. WoolfordDOI:10.1139/v62-280日期:1962.9.1
The first successful preparation of ω,ω′-dibromides from the electrolyses of a series of ω-bromocarboxylic acids, Br(CH2)nCOOH (n = 5 to n = 11), is reported. Under conditions of fairly low temperature and current density, yields from 54 to 71% of these dibromides were obtained.The electrolysis of 11-bromoundecanoic acid is discussed as an example of how a small change in experimental conditions can produce a considerable change in the products of reaction. At 50°, the product was mainly 1,20-dibromoeicosane, whereas at 65° the products were mainly the esters methyl 11-bromoundecanoate and methyl 11-methoxyundecanoate.
-
Synthesis of Alkylene-Bridged Diphenyl-Oligoynes作者:Christian Klinger、Otto Vostrowsky、Andreas HirschDOI:10.1002/ejoc.200500851日期:2006.3Alkylene chains with up to 40 methylene groups were employed. The two terminal acetylene functions were introduced prior to the oligoyne elongation by twofold introduction of additional C2-acetylene or C4-butadiyne building blocks. The final step was the intramolecular acetylene coupling upon which very large macrocycles with up to 62 ring members containing segregated sp-, sp2- and sp3-segments were formed
-
Intramolecular Reactions of Tethered Furan‐Based Bis( <i>p</i> ‐quinodimethanes)作者:Douglas A. Klumpp、Thomas M. Gilbert、Walter S. TrahanovskyDOI:10.1002/ejoc.201600995日期:2016.11generated in the gas phase by flash vacuum pyrolysis (FVP) of diester precursors. These furan-based bis(p-quinodimethanes) are shown to undergo reactions leading to macrocycles. The observed products strongly support a mechanism involving cyclic diradical intermediates. Formation of the furan-based p-quinodimethane and the corresponding cyclization chemistry was studied by high-level ab initio calculations
表征谱图
-
氢谱1HNMR
-
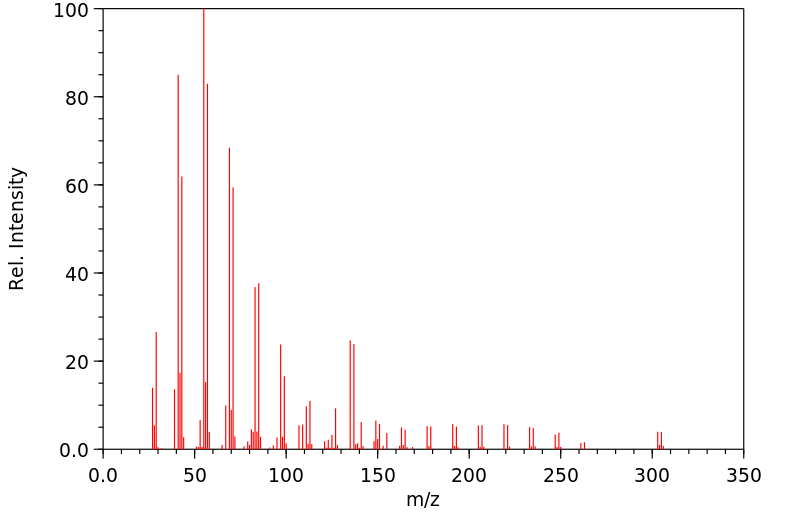
质谱MS
-
碳谱13CNMR
-
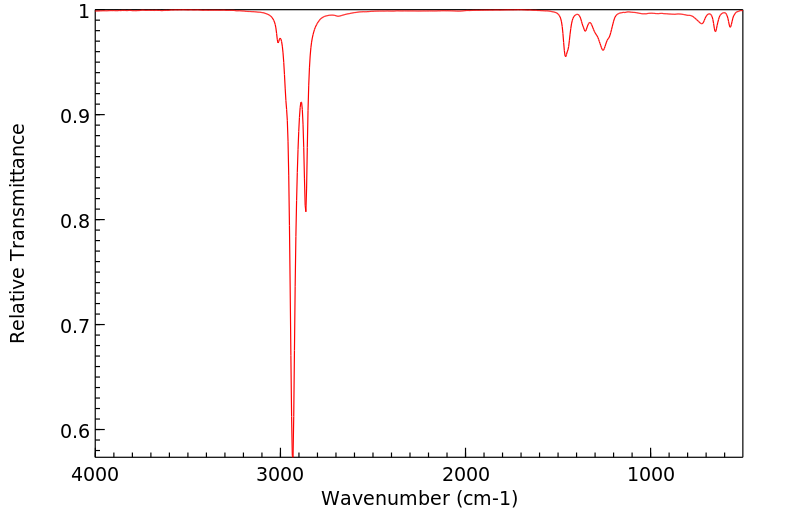
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4