1,3,5-己三烯 | 821-07-8
中文名称
1,3,5-己三烯
中文别名
——
英文名称
(E)-1,3,5-hexatriene
英文别名
1,3,5-hexatriene;trans-1,3,5-hexatriene;(E)-hexa-1,3,5-triene;trans-hexatriene;(E)-hexatriene;(3E)-hexa-1,3,5-triene
CAS
821-07-8
化学式
C6H8
mdl
——
分子量
80.1295
InChiKey
AFVDZBIIBXWASR-AATRIKPKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-12°C
-
沸点:80.15°C
-
密度:0.7369
-
蒸汽压力:89.93 mmHg
-
大气OH速率常数:5.56e-11 cm3/molecule*sec
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3,5-已三烯 1,3,5-hexatriene 2235-12-3 C6H8 80.1295 —— (Z)-1,3,5-hexatriene 2612-46-6 C6H8 80.1295 间戊二烯 penta-1,3-diene 504-60-9 C5H8 68.1185 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (Z)-1,3,5-hexatriene 2612-46-6 C6H8 80.1295 2,4-已二烯 (2E,4E)-hexa-2,4-diene 5194-51-4 C6H10 82.1454 2,4-已二烯 (E,Z)-2,4-hexadiene 5194-50-3 C6H10 82.1454 —— trans-1,2,4,6-Heptatetraen 69961-24-6 C7H8 92.1405 —— cis-1,3-hexadiene 14596-92-0 C6H10 82.1454 1,3-己二烯 1,3-hexadiene 20237-34-7 C6H10 82.1454
反应信息
-
作为反应物:参考文献:名称:Alder; v. Brachel, Justus Liebigs Annalen der Chemie, 1957, vol. 608, p. 195,198摘要:DOI:
-
作为产物:描述:参考文献:名称:生物质原料的脱氧:糖和糖醇的氧化or催化脱氧摘要:将糖转变为油:糖部分的脱氧反应对于将生物质转化为化学物质和燃料非常重要。使用另一种醇作为溶剂/还原剂,成功地将甲基三氧合or催化的脱氧脱水反应应用于此目的。该反应是高度立体,得到线性多烯的产品选自C 4 -C 6糖醇和芳族化合物选自C 4 -C 6克的糖。DOI:10.1002/anie.201203877
文献信息
-
Acyldemetallation of titanium(III) π-allylic complexes作者:A.N. Kasatkin、A.N. Kulak、G.A. TolstikovDOI:10.1016/0022-328x(88)87004-9日期:1988.5Titanium(III) π-allylic complexes, prepared by the interaction of 1,3-dienes or trienes with Cp2TiCl2 and n-PrMgBr, react with carboxylic acid chlorides RCOCl (R = alkyl, aryl, alkenyl) to give β, γ-unsaturated ketones in high yields. The reaction takes place at the most substituted carbon atom of the π-allylic ligand.
-
Expanding the Scope of Biomass-Derived Chemicals through Tandem Reactions Based on Oxorhenium-Catalyzed Deoxydehydration作者:Mika Shiramizu、F. Dean TosteDOI:10.1002/anie.201307564日期:2013.12.2New modes of DODH: Oxorhenium compounds act as deoxydehydration(DODH)/acid dual‐purpose catalysts to transform biomass‐derived diol substrates into a variety of commodity chemical precursors. The power of this approach is highlighted by a tandem [1,3]‐OH shift/DODH of 2‐ene‐1,4‐diols and 2,4‐diene‐1,6‐diols, and by a DODH/esterification sequence of sugar acids to unsaturated esters for the production
-
Carbene-carbene rearrangements as a route to 1,5-dihydropentalene作者:Udo H. Brinker、Ilona FleischhauerDOI:10.1016/0040-4020(81)80016-6日期:1981.1adducts of 4, 5a and 5b were obt in the reaction with perfluorobut-2-yne. The formation of 1,5-dihydropentalene 4 is explained by a double ring expansion sequence involving consecutive carbene-carbene rearrangements with 1,3-carbon and subsequent 1,2-hydrogen shifts, supported by the reaction of double labelled (13C-depleted) 3. From readily available 3 at low temperatures formation and fusion of two
-
Reaction of 1,3-Dienes with Nucleophiles Catalysed by [1,2-Bis(dialkylphosphino)-ethane] palladium Complexes作者:P. W. Jolly、N. KokelDOI:10.1055/s-1990-27010日期:——A(η2-butadiene)[1,2-bis(dialkylphosphino)ethane]palladium complex catalyses the reaction of 1,3-dienes with active hydrogen compounds or alcohols to give 1:1 adducts in high yield under moderate conditions.
-
Interaction of vinylpyridines with 1,3-dienes catalyzed by transition metal complexes作者:F. A. Selimov、O. A. Ptashko、A. A. Fatykhov、N. R. Khalikova、U. M. DzhemilevDOI:10.1007/bf00698950日期:1993.5The linear and cyclic cooligomerization of 2-vinyl-, 2-methyl-5-vinyl-, and 4-vinylpyridines with 1,3-dienes and trienes catalyzed by complexes of transition metals (Fe, Co, Ni, Mn, Cr, Pd, Ru, Rh, and Zr) was carried out to give unsaturated pyridines containing alkenyl and cycloalkenyl substituents.
表征谱图
-
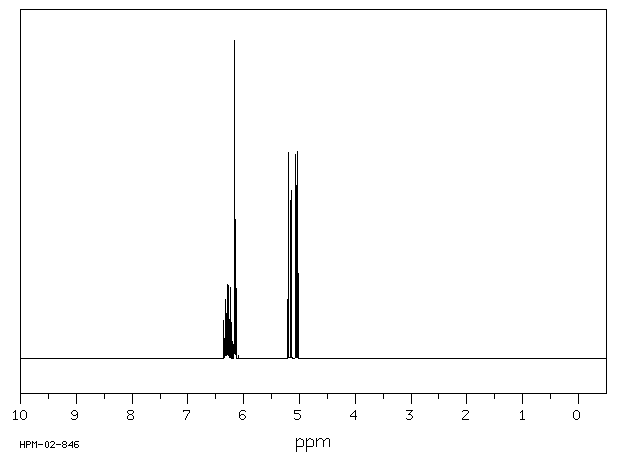
氢谱1HNMR
-
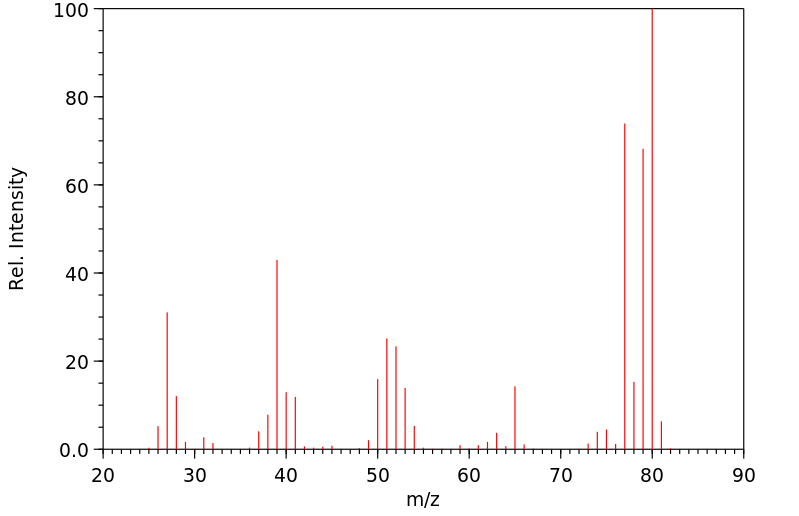
质谱MS
-
碳谱13CNMR
-
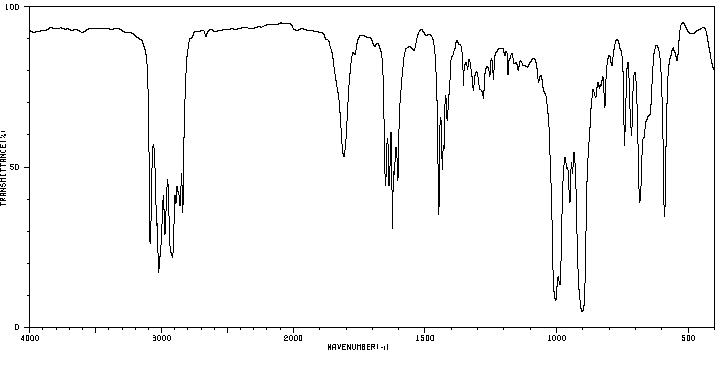
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-