1-溴-4-甲基萘 | 6627-78-7
中文名称
1-溴-4-甲基萘
中文别名
4-溴-1-甲基萘;1-甲基-4-溴萘
英文名称
1-bromo-4-methylnaphthalene
英文别名
4-bromo-1-methylnaphthalene
CAS
6627-78-7
化学式
C11H9Br
mdl
——
分子量
221.096
InChiKey
IDRVLLRKAAHOBP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127-128℃
-
沸点:162-164 °C/12 mmHg (lit.)
-
密度:1.419 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2903999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将产品存放在密闭、阴凉干燥的地方保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-Bromo-4-methylnaphthalene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Bromo-4-methylnaphthalene
CAS number: 6627-78-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H9Br
Molecular weight: 221.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-Bromo-4-methylnaphthalene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Bromo-4-methylnaphthalene
CAS number: 6627-78-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H9Br
Molecular weight: 221.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-溴代萘 1-Bromonaphthalene 90-11-9 C10H7Br 207.07 甲基萘 1-Methylnaphthalene 90-12-0 C11H10 142.2 1,4-二溴代萘 1,4-dibromonaphthalene 83-53-4 C10H6Br2 285.966 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(氯甲基)-4-溴萘 1-(chloromethyl)-4-bromonaphthalene 101828-11-9 C11H8BrCl 255.542 4-溴-1-萘醛 4-bromo-1-naphthaldehyde 50672-84-9 C11H7BrO 235.08 1-乙基-4-溴萘 1-bromo-4-ethylnaphthalene 67668-17-1 C12H11Br 235.123 —— 1-bromo-4-vinylnaphthalene 74839-80-8 C12H9Br 233.107 4-溴-1-萘甲腈 4-bromo-1-naphthonitrile 92616-49-4 C11H6BrN 232.079 1-溴-4-(溴甲基)萘 1-bromo-4-bromomethyl-naphthalene 79996-99-9 C11H8Br2 299.993 —— (4-bromonaphthalen-1-yl)methanamine 578029-11-5 C11H10BrN 236.111 2,4-二溴-1-甲基萘 1-methyl-2,4-dibromonaphthalene 3278-84-0 C11H8Br2 299.993 1-溴-4-(二溴甲基)萘 1-bromo-4-(dibromomethyl)naphthalene 1008361-70-3 C11H7Br3 378.889 —— 4-bromo-1-naphthalenecarboxaldehyde oxime 664364-16-3 C11H8BrNO 250.095 1-溴-4-(三氟甲基)萘 1-bromo-4-(trifluoromethyl)naphthalene 37827-77-3 C11H6BrF3 275.068 4-溴-1-萘甲酸 4-bromonaphthalene-1-carboxylic acid 16650-55-8 C11H7BrO2 251.079 4-溴-1-萘甲酸甲酯 methyl 4-bromo-1-naphthoate 35615-97-5 C12H9BrO2 265.106 甲基萘 1-Methylnaphthalene 90-12-0 C11H10 142.2 —— 2-(4-bromonaphthalen-1-yl)-1,3-dioxolane 901272-98-8 C13H11BrO2 279.133 4-溴-1-萘甲酸乙酯 ethyl 4-bromo-1-naphthoate 51934-43-1 C13H11BrO2 279.133 —— 3,10-dimethyl-perylene 25889-65-0 C22H16 280.369 1,4-二甲基萘 1,4-dimethylnaphthalene 571-58-4 C12H12 156.227 - 1
- 2
反应信息
-
作为反应物:描述:1-溴-4-甲基萘 在 乙醇 、 (4-Ph)Triaz(NHPiPr2)2Mn(CO)2Br 、 potassium tert-butylate 作用下, 以 甲苯 为溶剂, 以67 %的产率得到甲基萘参考文献:名称:锰催化芳基溴与乙醇的转移加氢脱溴摘要:报道了使用乙醇作为氢源的方便的 PNP 钳形锰催化溴化物转移氢化为相应的脱溴芳基化合物。生物质衍生乙醇的应用突出了该方法的可持续性。DOI:10.1002/ejoc.202201376
-
作为产物:描述:参考文献:名称:N-溴代琥珀酰亚胺在取代烷基芳烃的超声和微波辅助溴化反应中的化学选择性不同摘要:各种烷基芳基与N-溴丁二酰亚胺在纯净或水中的超声和微波辅助溴化反应显示出不同的化学选择性。因此,在超声波作用下,水中发生环取代,而在微波作用下,侧链α-溴化和环取代都发生。对于纯净的反应物,微波辅助反应中侧链α-溴化反应占主导地位。在水的存在下,微波促进的溴化的化学选择性类似于使用传统方法观察到的化学选择性。DOI:10.1016/j.tetlet.2007.03.023
文献信息
-
Substituted imidazol-pyridazine derivatives申请人:——公开号:US20030229096A1公开(公告)日:2003-12-11The present invention relates to compounds of formula 1 wherein A is an unsubstituted or substituted cyclic group; and R is hydrogen or lower alkyl; or a pharmaceutically acceptable acid addition salt thereof. These compounds are NMDA NR-2B receptor subtype specific blockers and are useful in the treatment of neurodegeneration, depression and pain.本发明涉及以下式的化合物 1 其中A是未取代或取代的环状基团;以及 R是氢或较低的烷基; 或其药学上可接受的酸盐。这些化合物是NMDA NR-2B受体亚型特异性阻断剂,对于治疗神经退行性疾病、抑郁症和疼痛具有用处。
-
Pd-Catalyzed Vinylation of Aryl Halides with Inexpensive Organosilicon Reagents Under Mild Conditions作者:Chu-Ting Yang、Jun Han、Jun Liu、Yi Li、Fan Zhang、Hai-Zhu Yu、Sheng Hu、Xiaolin WangDOI:10.1002/chem.201802573日期:2018.7.20Pd‐catalyzed Hiyama vinylation reaction of non‐activated aryl chlorides and bromides under mild conditions was developed. The use of efficient vinyl donors and electron‐rich sterically hindered phosphine ligands was critical for the success of the reaction. The products of this transformation can be used for Am/Cm separation, an important challenge in nuclear fuel reprocessing. The substituent effect
-
An Opportunity for Mg-Catalyzed Grignard-Type Reactions: Direct Coupling of Benzylic Halides with Pinacolborane with 10 mol % of Magnesium作者:Christine Pintaric、Sandra Olivero、Yves Gimbert、Pierre Y. Chavant、Elisabet DuñachDOI:10.1021/ja1052973日期:2010.9.1Mg in catalytic amounts as the only metal permits the reductive coupling between benzyl halides and pinacolborane. HBpin acts both as an electrophile and as a reducing agent to regenerate an organomagnesium species in situ. An hydride oxidation mechanism is proposed on the basis of DFT calculations.
-
PROCESS FOR PREPARING BORONIC ACIDS AND ESTERS IN THE PRESENCE OF MAGNESIUM METAL申请人:Dunach Isabel公开号:US20110282090A1公开(公告)日:2011-11-17The present invention relates to the process for preparing a boronic acid or ester chemically, in which an aromatic compound is reacted with a boronating agent, in the presence of magnesium metal (Mg 0 ). The invention also relates to the boronic acids or esters that can be obtained by means of this process and to the use thereof for example as a synthesis intermediate, in the Suzuki reaction, in the pharmaceutical or alternatively electronics field.
-
Photochemistry of substituted 1-naphthylmethyl esters of phenylacetic and 3-phenylpropanoic acid: radical pairs, ion pairs, and Marcus electron transfer作者:Dayal P. DeCosta、James A. PincockDOI:10.1021/ja00059a012日期:1993.3The ring-substituted 1-naphthylmethyl esters of phenylacetic (3a-k) and 3-phenylpropanoic (5a-c) acid have been photolyzed in methanol solvent. The major products of these reactions are derived from two critical intermediates, the 1-naphthylmethyl radical/acyloxy radical pair and the 1-naphthylmethyl cation/carboxylate anion ion pair. The radical pair results in formation of the in-cage coupled products
表征谱图
-
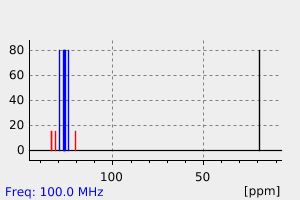
氢谱1HNMR
-
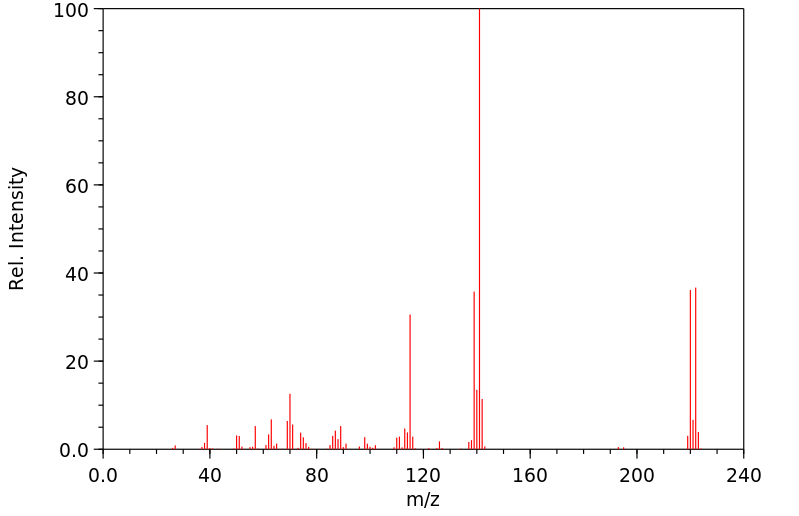
质谱MS
-
碳谱13CNMR
-
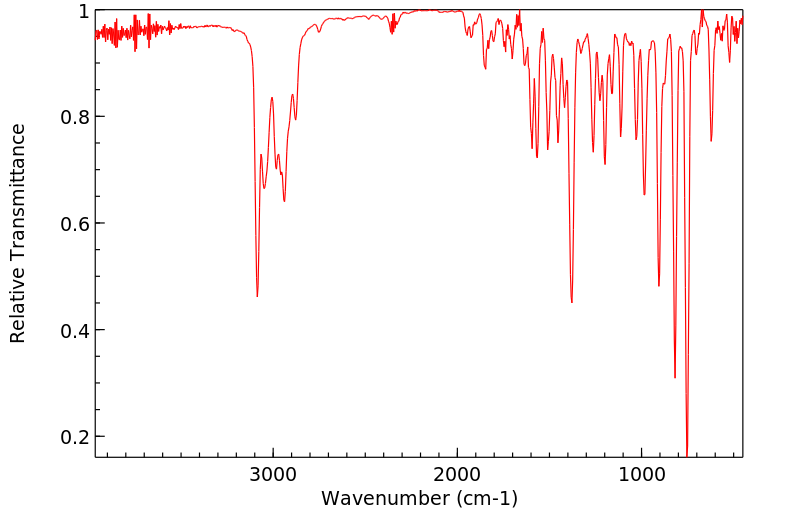
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮