2,6-二甲氧基苯甲酸 | 1466-76-8
中文名称
2,6-二甲氧基苯甲酸
中文别名
2,6-二甲氧基苯酸;雷锁酸二甲醚
英文名称
2-6-dimethoxybenzoic acid
英文别名
2,6-Dimethoxybenzoic acid
CAS
1466-76-8
化学式
C9H10O4
mdl
MFCD00002437
分子量
182.176
InChiKey
MBIZFBDREVRUHY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:185-187 °C (lit.)
-
沸点:275.56°C (rough estimate)
-
密度:1.2481 (rough estimate)
-
溶解度:14.7g/l可溶
-
LogP:0.660
-
物理描述:Solid
-
蒸汽压力:1.90e-07 mmHg
-
保留指数:1460
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知危险反应。应避免与氧化物、碱接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:55.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S26,S36,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2918990090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:DG8598725
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:保持贮藏器密封,储存在阴凉、干燥处,并确保工作间有良好的通风或排气装置。
SDS
| Name: | 2 6-Dimethoxybenzoic Acid 99% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 1466-76-8 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1466-76-8 | 2,6-Dimethoxybenzoic Acid | 99 | 215-985-4 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1466-76-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white to off-white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 186.00 - 187.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: 35 wt% (25 c)
Specific Gravity/Density:
Molecular Formula: C9H10O4
Molecular Weight: 182.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1466-76-8: DG8598725 LD50/LC50:
CAS# 1466-76-8: Oral, rat: LD50 = >500 mg/kg.
Carcinogenicity:
2,6-Dimethoxybenzoic Acid - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1466-76-8: No information available.
Canada
CAS# 1466-76-8 is listed on Canada's NDSL List.
CAS# 1466-76-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1466-76-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
生物活性
2,6-二甲氧基苯甲酸是一种在植物来源的食物中发现的多酚化合物。
化学性质本品为固体,熔点186~187℃,难溶于水,但能溶于碱及有机溶剂,如苯、甲苯。
用途2,6-二甲氧基苯甲酸是杀螨剂苯螨特的中间体,并且用作医药中间体和有机合成原料。
生产方法该化合物可通过三步反应合成:
-
第一步:以N,N-二甲基甲酰胺(DMF)为溶剂,间苯二酚∶碳酸钾∶二氧化碳=1.0∶0.5∶1.5,在1.3MPa、135~137℃条件下反应16.5小时后,经酸化制得2,6-二羟基苯甲酸。
-
第二步:使2,6-二羟基苯甲酸∶硫酸二甲酯=1∶3,在碱性条件下反应得到2,6-二羟基苯甲酸甲酯。
-
第三步:将上一步产物经碱催化水解、酸化,最终制得2,6-二甲氧基苯甲酸。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 2,6-dimethoxybenzoate 1464-96-6 C11H14O4 210.23 2,6-二羟基苯甲酸 2,6-Dihydroxybenzoic acid 303-07-1 C7H6O4 154.122 2,6-二甲氧基苯甲醛 2,6-dimethoxybenzaldehyde 3392-97-0 C9H10O3 166.177 —— 3-Ethylpentan-3-yl 2,6-dimethoxybenzoate 336621-33-1 C16H24O4 280.364 2,6-二甲氧基苯乙酮 2,6-dimethoxyacetophenone 2040-04-2 C10H12O3 180.203 2,6-二甲氧基苯甲酰氯 2,6-dimethoxybenzoyl chloride 1989-53-3 C9H9ClO3 200.622 2,6-二甲氧基甲苯 2,6-dimethoxytoluene 5673-07-4 C9H12O2 152.193 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-甲氧基水杨酸 2-hydroxy-6-methoxybenzoic acid 3147-64-6 C8H8O4 168.149 2,6-二甲氧基苯甲酸甲酯 methyl 2,6-dimethoxybenzoate 2065-27-2 C10H12O4 196.203 —— ethyl 2,6-dimethoxybenzoate 1464-96-6 C11H14O4 210.23 —— vinyl 2,6-dimethoxybenzoate —— C11H12O4 208.214 2,6-二甲氧基苯甲酸苄酯 benzyl 2,6-dimethoxybenzoate 34328-54-6 C16H16O4 272.301 —— 2-hydroxyethyl 2,6-dimethoxybenzoate 13084-23-6 C11H14O5 226.229 2,6-二羟基苯甲酸 2,6-Dihydroxybenzoic acid 303-07-1 C7H6O4 154.122 (2,6-二甲氧基苯基)甲醇 2,6-dimethoxybenzyl alcohol 16700-55-3 C9H12O3 168.192 —— butyl 2,6-dimethoxybenzoate 170169-95-6 C13H18O4 238.284 —— 2,6-dimethoxybenzoic anhydride 2638-22-4 C18H18O7 346.337 2,6-二甲氧基苯甲醛 2,6-dimethoxybenzaldehyde 3392-97-0 C9H10O3 166.177 —— phenyl 2,6-dimethoxybenzoate 443734-79-0 C15H14O4 258.274 —— acetic2,6-dimethoxybenzoic anhydride —— C11H12O5 224.213 —— 4-methoxyphenyl 2,6-dimethoxybenzoate 443893-74-1 C16H16O5 288.3 —— 2,6-Dimethoxy-4-trifluoromethyl-benzoic acid 1236303-15-3 C10H9F3O4 250.174 —— 2,6-dihydroxybenzoic acid phenylethyl ester —— C17H18O4 286.328 —— (E)-styryl 2,6-dimethoxybenzoate 1262967-38-3 C17H16O4 284.312 —— 3-iodo-6-methoxysalicylic acid 113452-76-9 C8H7IO4 294.046 —— 4-bromophenyl 2,6-dimethoxybenzoate 415694-05-2 C15H13BrO4 337.17 3-溴-2-羟基-6-甲氧基水杨酸 5-bromo-6-hydroxy-2-methoxybenzoic acid 84225-86-5 C8H7BrO4 247.045 —— (2R)-hexyl-2',6'-dimethoxybenzoate —— C15H22O4 266.337 3-氯-2,6-二甲氧基苯甲酸 3-chloro-2,6-dimethoxybenzoic acid 36335-47-4 C9H9ClO4 216.621 2,6-二羟基苯甲酸甲酯 methyl 2,6-dihydroxybenzoate 2150-45-0 C8H8O4 168.149 3-溴-2,6-二甲氧基苯甲酸 3-bromo-2,6-dimethoxybenzoic acid 73219-89-3 C9H9BrO4 261.072 3,5-二氯-2,6-二甲氧基苯甲酸 2,6-dimethoxy-3,5-dichlorobenzoic acid 73219-91-7 C9H8Cl2O4 251.066 3,5-二溴-2,6-二甲氧基苯甲酸 3,5-Dibromo-2,6-dimethoxybenzoic acid 73219-90-6 C9H8Br2O4 339.968 —— 3,5-Dibrom-2-hydroxy-6-methoxy-benzoesaeure 90005-24-6 C8H6Br2O4 325.941 —— 2,6-dimethoxybenzyl isopropyl ether 109682-58-8 C12H18O3 210.273 —— 2-(2,2-Difluorocyclopropyl)ethyl 2,6-dimethoxybenzoate 117388-11-1 C14H16F2O4 286.275 2,6-二甲氧基-苯甲酰胺 2,6-dimethoxybenzamide 21864-67-5 C9H11NO3 181.191 —— Acetic acid 2,6-dimethoxy-benzyl ester 67698-76-4 C11H14O4 210.23 2,6-二甲氧基苯甲酰基氟化物 2,6-dimethoxybenzoyl fluoride 130161-08-9 C9H9FO3 184.167 2,6-二甲氧基苯乙酮 2,6-dimethoxyacetophenone 2040-04-2 C10H12O3 180.203 2,6-二甲氧基苯甲酰氯 2,6-dimethoxybenzoyl chloride 1989-53-3 C9H9ClO3 200.622 —— 1,4-dioxan-2-yl 2,6-dimethoxybenzoate 1610377-96-2 C13H16O6 268.266 2,6-二甲氧基甲苯 2,6-dimethoxytoluene 5673-07-4 C9H12O2 152.193 —— (5-oxotetrahydrofuran-2-yl)methyl 2,6-dimethoxybenzoate —— C14H16O6 280.277 —— 2,6-dimethoxybenzohydroxamic acid 51410-99-2 C9H11NO4 197.191 —— 3-bromo-2,6-dimethoxybenzyl alcohol 586392-15-6 C9H11BrO3 247.089 —— 2',6'-dimethoxypropiophenone 3840-02-6 C11H14O3 194.23 —— 2,6-dihydroxybenzoic acid phenyl ethyl ester —— C15H14O4 258.274 —— (2,6-dimethoxyphenyl)-N,N-dimethylcarboxamide 71955-53-8 C11H15NO3 209.245 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:2,6-二甲氧基苯甲酸 在 potassium tert-butylate 、 氧气 、 copper diacetate 、 silver carbonate 作用下, 以 乙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 18.0h, 生成 1,2,3-三甲氧基苯参考文献:名称:芳香羧酸的脱羧醚化摘要:脱羧 Chan-Evans-Lam 型偶联是一种从容易获得的芳族羧酸开始区域特异性构建二芳基和烷基芳基醚的新策略。它们允许在有氧条件下,在碳酸银作为脱羧催化剂和乙酸铜作为交叉偶联催化剂存在下,通过与原硅酸烷基酯或硼酸芳基酯反应,将各种芳族羧酸盐转化为相应的芳基醚。DOI:10.1021/ja304539j
-
作为产物:描述:参考文献:名称:Lobry de Bruyn, Recueil des Travaux Chimiques des Pays-Bas, 1883, vol. 2, p. 237摘要:DOI:
-
作为试剂:描述:参考文献:名称:金(I)催化的原脱羧机理摘要:描述了金催化的原脱羧反应的机理研究。已通过实验和计算研究了每个反应步骤。更具体地说,已经通过动力学研究确定了用于脱羧步骤的活化参数。关于芳基金中间体水解的进一步实验研究表明,原脱脂可以在高转化率下与脱羧过程竞争。速率限制步骤中的此切换已显示为取决于p K a。这些研究得到了DFT计算的支持,使人们能够更好地了解反应机理的哪些普遍特征是造成脱羧过程的原因。DOI:10.1002/chem.201405716
文献信息
-
[EN] DISUBSTITUTED OCTAHY-DROPYRROLO [3,4-C] PYRROLES AS OREXIN RECEPTOR MODULATORS<br/>[FR] OCTAHYDROPYRROLO [3,4-C] PYRROLES DISUBSTITUÉS UTILISÉS COMME MODULATEURS DU RÉCEPTEUR DE L'OREXINE申请人:JANSSEN PHARMACEUTICA NV公开号:WO2012145581A1公开(公告)日:2012-10-26Disubstituted octahydropyrrolo[3,4-c]pyrrole compounds are described, which are useful as orexin receptor modulators. Such compounds may be useful in pharmaceutical compositions and methods for the treatment of diseased states, disorders, and conditions mediated by orexin activity, such as insomnia.
-
AZOLE INHIBITORS OF CYTOKINE PRODUCTION
-
Dimethylmalonyltrialkylphosphoranes: probing the steric effect on phosphorus and its stereochemical consequence in esterification reactions of chiral secondary alcohols作者:J. Dyck、S. Zavorine、A.J. Robertson、A. Capretta、V. Larichev、J. Britten、J. McNultyDOI:10.1016/j.jorganchem.2004.10.046日期:2005.5High chemical yields and high levels of stereochemical inversion are demonstrated in the phosphorane-mediated esterification reaction of chiral alcohols with non-hindered carboxylic acids through the incorporation of sterically non-hindered alkyl groups of phosphorus.
-
Dimethylmalonyltrialkylphosphoranes: New General Reagents for Esterification Reactions Allowing Controlled Inversion or Retention of Configuration on Chiral Alcohols作者:James McNulty、Alfredo Capretta、Vladimir Laritchev、Jeff Dyck、Al J. RobertsonDOI:10.1021/jo026639y日期:2003.2.1through reaction of a trialkylphosphine with 2-chlorodimethylmalonate in the presence of triethylamine. These new reagents promote the condensation reaction of carboxylic acids with alcohols to provide esters along with trialkylphosphine oxide and dimethylmalonate. The condensation reaction of chiral secondary alcohols can be controlled to give either high levels of inversion or retention through a
-
[EN] BORONIC ACID DERIVATIVES AND THERAPEUTIC USES THEREOF<br/>[FR] DÉRIVÉS D'ACIDE BORONIQUE ET LEURS UTILISATIONS THÉRAPEUTIQUES申请人:REMPEX PHARMACEUTICALS INC公开号:WO2016003929A1公开(公告)日:2016-01-07Disclosed herein are antimicrobial compounds compositions, pharmaceutical compositions, the use and preparation thereof. Some embodiments relate to boronic acid derivatives and their use as therapeutic agents.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
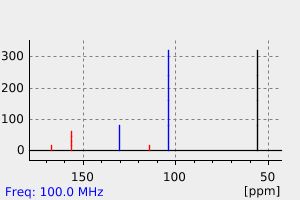
碳谱13CNMR
-
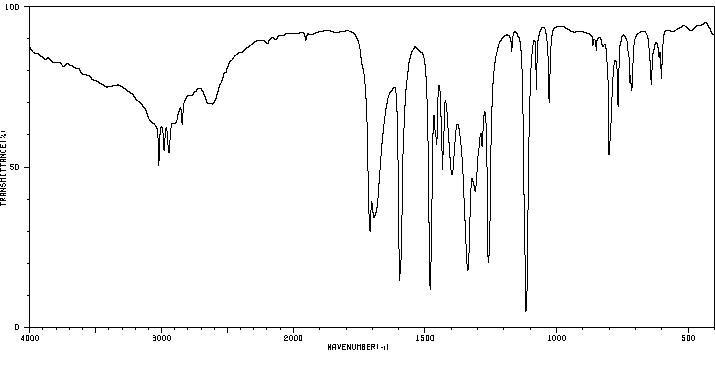
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫