2-(正庚酰)噻吩 | 30711-40-1
中文名称
2-(正庚酰)噻吩
中文别名
2-(庚酰)噻吩
英文名称
2-thienyl hexyl ketone
英文别名
1-thiophen-2-yl-heptan-1-one;1-[2]thienyl-heptan-1-one;1-[2]Thienyl-heptan-1-on;n-Hexyl-2-thienyl-keton;2-(n-Heptanoyl)thiophene;1-thiophen-2-ylheptan-1-one
CAS
30711-40-1
化学式
C11H16OS
mdl
——
分子量
196.313
InChiKey
BDNFJEMAAFFMFH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:154-156°C 15mm
-
密度:1.026
-
闪点:154-156°C/15mm
-
LogP:3.817 (est)
-
保留指数:1565
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:13
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.55
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
海关编码:2934999090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-(N-庚基)噻吩 2-n-heptylthiophene 18794-78-0 C11H18S 182.33 —— 5-Heptyl-2-thiophencarbaldehyd 98790-79-5 C12H18OS 210.34
反应信息
-
作为反应物:参考文献:名称:Buu-Hoi et al., Journal of the Chemical Society, 1955, p. 1581,1583摘要:DOI:
-
作为产物:参考文献:名称:摘要:DOI:
文献信息
-
Dimethylaluminum methaneselenolate—a useful reagent for the preparation of selenoesters. A new friedel-crafts acylation procedure promoted by Cu(I)作者:Alan P. Kozikowski、Anthony AmesDOI:10.1016/s0040-4020(01)96721-3日期:1985.1The preparation of a new aluminum reagent, dmiethylaluminum methaneselenolate (Me2AlSeMe) is described. The reactivity of this aluminum reagent toward a variety of organic substrates has been studied. Me2AlSeMe will convert O-alkyl esters to selenoesters in high yield. These selenoesters function as extremely reactive acyl transfer agents and are converted to acids, esters, and amides on reaction with
-
Process for producing 2-acylthiophene compound申请人:Bando Seiji公开号:US20070149787A1公开(公告)日:2007-06-28The present invention provides a process for producing a 2-acylthiophene compound which has a low content of the 3-isomer generated as a by-product, the process comprising reacting a thiophene compound represented by formula (1): wherein R 1 is a hydrogen atom, a C 1-6 alkyl group, a phenyl group, or a halogen atom, with at least one member selected from the group consisting of acid anhydrides represented by formula (2): wherein R 2 is a C 1-6 alkyl group or a phenyl group, and acid halides represented by formula (3): wherein R 2 is as defined above and X is a halogen atom, in the presence of a solid acid catalyst at a temperature less than 75° C. in the absence of solvent, thus producing a 2-acylthiophene compound represented by formula (4): wherein R 1 and R 2 are as defined above.
-
Direct Acylation of Unactivated Alkyl Halides with Aldehydes through N‐Heterocyclic Carbene Organocatalysis作者:Qing‐Zhu Li、Rong Zeng、Peng‐Shuai Xu、Xin‐Hang Jin、Chuan Xie、Qi‐Chun Yang、Xiang Zhang、Jun‐Long LiDOI:10.1002/anie.202309572日期:2023.10.2with unactivated alkyl halides in the presence of an NHC organocatalyst, thus enabling the rapid synthesis of various ketones from readily available starting materials under mild conditions. This method was also applied to the late-stage functionalization of pharmaceutical derivatives. Mechanistic investigations suggest a closed-shell nucleophilic substitution mechanism for this organocatalytic reaction在 NHC 有机催化剂存在下,醛与未活化的卤代烷发生交叉偶联,从而能够在温和条件下从容易获得的起始材料快速合成各种酮。该方法也应用于药物衍生物的后期功能化。机理研究表明该有机催化反应存在闭壳亲核取代机制。
-
Polyhydroxyalkanoate containing unit with thienyl structure in the side chain, process for its production, charge control agent, toner binder and toner which contain this polyhydroxyalkanoate, and image-forming method and image-forming apparatus which make use of the toner申请人:CANON KABUSHIKI KAISHA公开号:EP1245605A2公开(公告)日:2002-10-02A polyhydroxyalkanoate characterized by having in the molecule a unit represented by Chemical Formula (1) : wherein n may assume any one integral value within the range of from 1 to 8. Also disclosed are a process for producing the polyhydroxyalkanoate by the use of a microorganism having the ability to produce the polyhydroxyalkanoate and accumulate it in the bacterial body; a charge control agent, a toner binder and a toner which contain this polyhydroxyalkanoate; and an image-forming method and an image-forming apparatus which make use of the toner.
-
PROCESS FOR PRODUCING 2-ACYLTHIOPHENE COMPOUND申请人:SUMITOMO SEIKA CHEMICALS CO., LTD.公开号:EP1695972A1公开(公告)日:2006-08-30The present invention provides a process for producing a 2-acylthiophene compound which has a low content of the 3-isomer generated as a by-product, the process comprising reacting a thiophene compound represented by formula (1) : wherein R1 is a hydrogen atom, a C1-6 alkyl group, a phenyl group, or a halogen atom, with at least one member selected from the group consisting of acid anhydrides represented by formula (2): wherein R2 is a C1-6 alkyl group or a phenyl group, and acid halides represented by formula (3): wherein R2 is as defined above and X is a halogen atom, in the presence of a solid acid catalyst at a temperature less than 75°C in the absence of solvent, thus producing a 2-acylthiophene compound represented by formula (4): wherein R1 and R2 are as defined above.
表征谱图
-
氢谱1HNMR
-
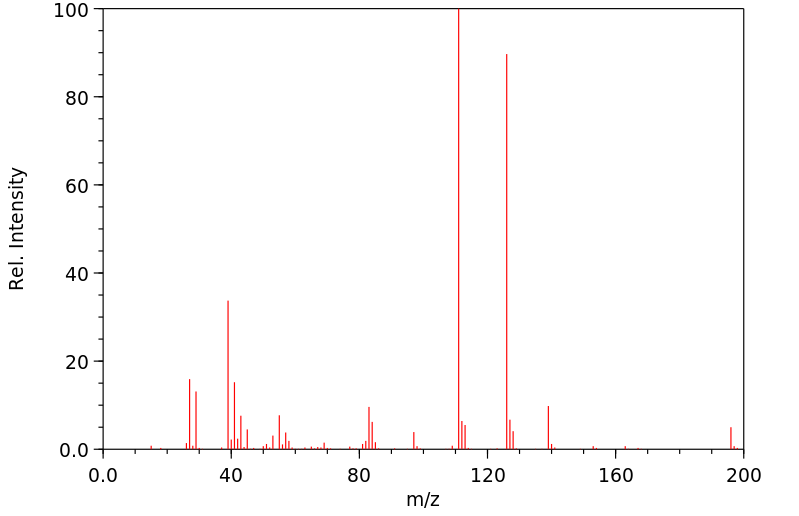
质谱MS
-
碳谱13CNMR
-
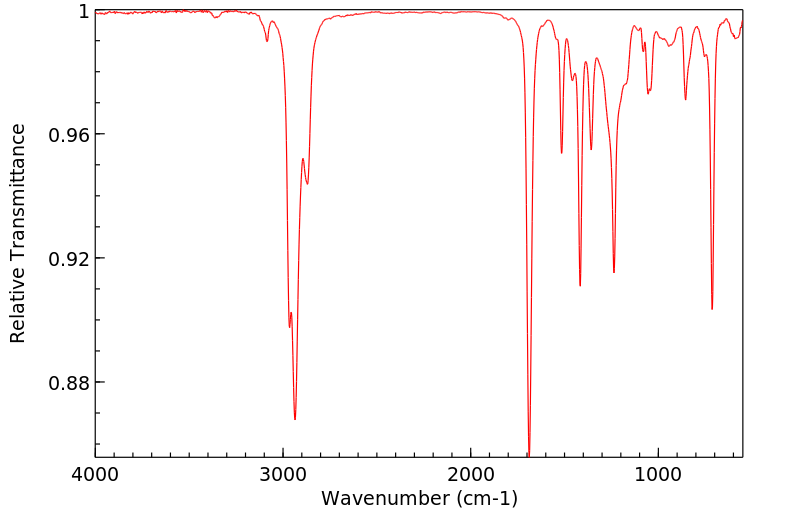
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷