2-呋喃甲基乙酯 | 614-99-3
中文名称
2-呋喃甲基乙酯
中文别名
2-呋喃甲酸乙酯;糖酸乙酯;呋喃酸乙酯;2-糖酸乙酯;糠酸乙酯;呋喃甲酸乙酯;焦黏酸乙酯;2-糠酸乙酯;Α-呋喃甲酸乙酯
英文名称
Ethyl 2-furoate
英文别名
ethyl 2-furancarboxylate;ethyl furan-2-carboxylate;ethyl furan‐2‐carboxylate;2-ethyl furoate
CAS
614-99-3;1335-40-6
化学式
C7H8O3
mdl
MFCD00003237
分子量
140.139
InChiKey
NHXSTXWKZVAVOQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:32-37 °C (lit.)
-
沸点:196 °C (lit.)
-
密度:1.117 g/mL at 25 °C (lit.)
-
闪点:158 °F
-
溶解度:Insoluble in water, soluble in alcohol and oils.
-
LogP:1.520
-
蒸汽压力:0.37 mmHg
-
保留指数:1056;1059;1056;1047;1045;1053;1046.8;1045;1062
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:10
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:39.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险品标志:F,F+
-
安全说明:S16
-
危险类别码:R11
-
WGK Germany:3
-
海关编码:29329990
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:LV1850000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-糠酸甲酯 2-furoic acid methyl ester 611-13-2 C6H6O3 126.112 5-溴-2-糠酸乙酯 ethyl 5-bromofuran-2-carboxylate 6132-37-2 C7H7BrO3 219.035 糠酸(呋喃甲酸) 2-Furoic acid 88-14-2 C5H4O3 112.085 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 正辛基-2-呋喃羧酸酯 octyl furan-2-carboxylate 39251-88-2 C13H20O3 224.3 —— 5-methyl-furan-2-carboxylic acid ethyl ester 14003-12-4 C8H10O3 154.166 5-羟基甲基呋喃-2-羧酸乙酯 ethyl 5-(hydroxymethyl)furan-2-carboxylate 76448-73-2 C8H10O4 170.165 5-甲酰基-2-呋喃甲酸乙酯 ethyl 5-formyl-2-furancarboxylate 22551-91-3 C8H8O4 168.149 —— ethyl 5-(ethoxymethyl)furan-2-carboxylate 1071696-43-9 C10H14O4 198.219 —— 5-methoxymethyl-furan-2-carboxylic acid ethyl ester 400868-44-2 C9H12O4 184.192 呋喃-2,5-二甲酸二乙酯 diethyl furan-2,5-dicarboxylate 53662-83-2 C10H12O5 212.202 —— 2-ethyl 5-methyl furan-2,5-dicarboxylate —— C9H10O5 198.175 —— ethyl 5-((formyloxy)methyl)furan-2-carboxylate 102653-88-3 C9H10O5 198.175 —— ethyl 5-chloro-2-fuorate 4301-39-7 C7H7ClO3 174.584 5-溴-2-糠酸乙酯 ethyl 5-bromofuran-2-carboxylate 6132-37-2 C7H7BrO3 219.035 —— ethyl 5-vinylfuroate 86471-36-5 C9H10O3 166.177 —— 5-ethyl-furan-2-carboxylic acid ethyl ester 62120-14-3 C9H12O3 168.192 5-氯甲基-2-呋喃甲酸乙酯 5-Chloromethyl-furan-2-carboxylic acid ethyl ester 2528-00-9 C8H9ClO3 188.611 —— ethyl 5-(bromomethyl)furan-2-carboxylate 74675-71-1 C8H9BrO3 233.062 3-溴-呋喃-2-羧酸乙酯 ethyl 3-bromofuran-2-carboxylate 32460-07-4 C7H7BrO3 219.035 糠酸(呋喃甲酸) 2-Furoic acid 88-14-2 C5H4O3 112.085 呋喃-2,5-二甲酸二甲酯 furan-2,5-dicarboxylic acid dimethyl ester 4282-32-0 C8H8O5 184.149 5-(氰基甲基)呋喃-2-羧酸乙酯 5-cyanomethyl-furan-2-carboxylic acid ethyl ester 51129-66-9 C9H9NO3 179.175 —— ethyl 5-((methylthio)methyl)furan-2-carboxylate 102439-44-1 C9H12O3S 200.258 —— 2-acetyl-5-furoic acid ethyl ester 13318-36-0 C9H10O4 182.176 —— Ethyl 5-tert-butylfuran-2-carboxylate 5398-05-0 C11H16O3 196.246 —— ethyl 5-<(dimethylamino)methyl>-2-furoate 100132-45-4 C10H15NO3 197.234 5-甲基-2-糠酸 5-methylfuran-2-carboxylic acid 1917-15-3 C6H6O3 126.112 5-(1-氯乙基)-2-糠酸乙酯 ethyl 5-(1-chloroethyl)furan-2-carboxylate 18744-04-2 C9H11ClO3 202.638 2,5-呋喃二甲酸 furan-2,5-dicarboxylic acid 3238-40-2 C6H4O5 156.095 5-乙酰基-2-呋喃甲酸甲酯 methyl 5-acetylfuran-2-carboxylate 13341-79-2 C8H8O4 168.149 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:FeCl3促进的酯的O-烷基裂解为羧酸摘要:已经开发了可靠且实用的FeCl 3促进的酯裂解程序。研究了包括TiCl 4,ZnO和FeCl 3等的路易斯酸作为羧酸酯O-烷基裂解的促进剂。在最佳反应条件下,发现FeCl 3(1.5当量)具有最高的活性,并能有效地增强芳基酯,烷基酯和芳族杂环酯的脱烷基作用,从而以54-98%的收率得到相应的羧酸,该方法提供了在中性条件下酯脱烷基的互补途径。版权所有©2011 John Wiley&Sons,Ltd.DOI:10.1002/aoc.1784
-
作为产物:参考文献:名称:Schwanert, Justus Liebigs Annalen der Chemie, 1860, vol. 116, p. 261摘要:DOI:
-
作为试剂:描述:参考文献:名称:医药激素中间体11-酮-16α,17α-环氧黄体酮的合成方法摘要:本发明公开了基于背景技术存在的技术问题,本发明提出了医药激素中间体11‑酮‑16α,17α‑环氧黄体酮的合成方法,包括如下步骤:在反应容器中加入2‑溴‑11α‑羟基‑14‑氨基‑16α,17α‑环氧黄体酮,硫酸钠溶液,控制搅拌速度,控制溶液温度,反应;加入呋喃甲酸乙酯溶液,三氯化锑粉末,升高溶液温度至,反应,加入氯化钾溶液,静置,析出晶体,过滤,用硝酸钠溶液洗涤多次,2,6‑二甲基吡啶溶液洗涤多次,二氟二氯甲烷溶液洗涤多次,在二甘醇甲乙醚溶液中重结晶,脱水剂脱水,得成品11‑酮‑16α,17α‑环氧黄体酮。公开号:CN108239137A
文献信息
-
A Bifunctional Copper Catalyst Enables Ester Reduction with H<sub>2</sub>: Expanding the Reactivity Space of Nucleophilic Copper Hydrides作者:Birte M. Zimmermann、Trung Tran Ngoc、Dimitrios-Ioannis Tzaras、Trinadh Kaicharla、Johannes F. TeichertDOI:10.1021/jacs.1c09626日期:2021.10.13activation of esters through hydrogen bonding and formation of nucleophilic copper(I) hydrides from H2, resulting in a catalytic hydride transfer to esters. The reduction step is further facilitated by a proton shuttle mediated by the guanidinium subunit. This bifunctional approach to ester reductions for the first time shifts the reactivity of generally considered “soft” copper(I) hydrides to previously
-
Oxidation of alcohols by transition metal complexes—iv作者:Ronald Grigg、Thomas R.B. Mitchell、Somyote SutthivaiyakitDOI:10.1016/0040-4020(81)85027-2日期:1981.1range of aldehydes with simple primary alcohols to give esters together with alcohols formed by reduction of the aldehydes. The proportion of ester can be increased by adding an efficient hydrogen acceptor. The reaction can also be used to produce 5- and 7-membered lactones from aromatic dialdehydes. Propan-2-ol and the in situ catalyst reduce some aromatic aldehydes to the corresponding alcohols withoutRhH(CO)PPh 3)3和由RhCl 3 ·3H 2 O,PPh 3和Na 2 CO 3原位制得的催化剂均催化多种醛与简单的伯醇反应生成酯以及由醛的还原。可以通过添加有效的氢受体来增加酯的比例。该反应也可用于由芳族二醛生产5元和7元内酯。丙-2-醇和原位催化剂可将一些芳香醛还原为相应的醇,而不会形成酯。
-
Preparation of amides mediated by isopropylmagnesium chloride under continuous flow conditions作者:Juan de M. Muñoz、Jesús Alcázar、Antonio de la Hoz、Ángel Díaz-Ortiz、Sergio-A. Alonso de DiegoDOI:10.1039/c2gc35037h日期:——mediated by Grignard reagents (the Bodroux reaction) is described. The procedure can be applied to a wide variety of primary and secondary amines and anilines, as well as to aromatic and aliphatic esters. The flow approach leads to improved yields and selectivities in the reaction, which has a sustainable purification procedure and a simple scale-up. This reaction represents an efficient and green alternative
-
COMPOUNDS AND USES THEREOF申请人:Yumanity Therapeutics, Inc.公开号:US20190330198A1公开(公告)日:2019-10-31The present invention features compounds useful in the treatment of neurological disorders. The compounds of the invention, alone or in combination with other pharmaceutically active agents, can be used for treating or preventing neurological disorders.本发明涉及用于治疗神经系统疾病的化合物。本发明的化合物可以单独或与其他药用活性剂结合使用,用于治疗或预防神经系统疾病。
-
Conversion of a metal–organic framework to N-doped porous carbon incorporating Co and CoO nanoparticles: direct oxidation of alcohols to esters作者:Yu-Xiao Zhou、Yu-Zhen Chen、Lina Cao、Junling Lu、Hai-Long JiangDOI:10.1039/c5cc01588j日期:——
A Co-based metal–organic framework undergoes pyrolysis to afford Co–CoO@N-doped porous carbon, which exhibits excellent catalytic performance toward the direct oxidation of alcohols to esters.
表征谱图
-
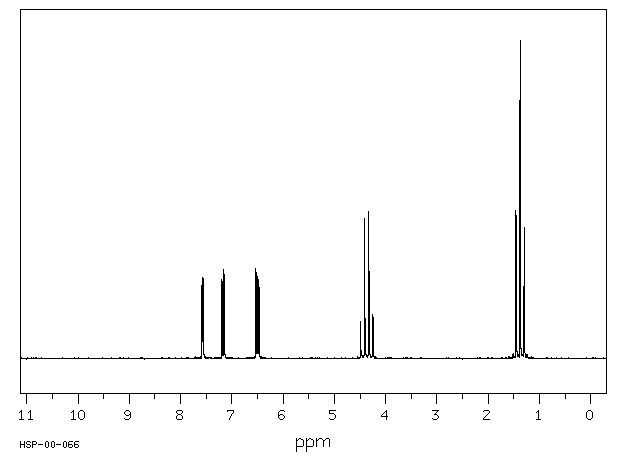
氢谱1HNMR
-
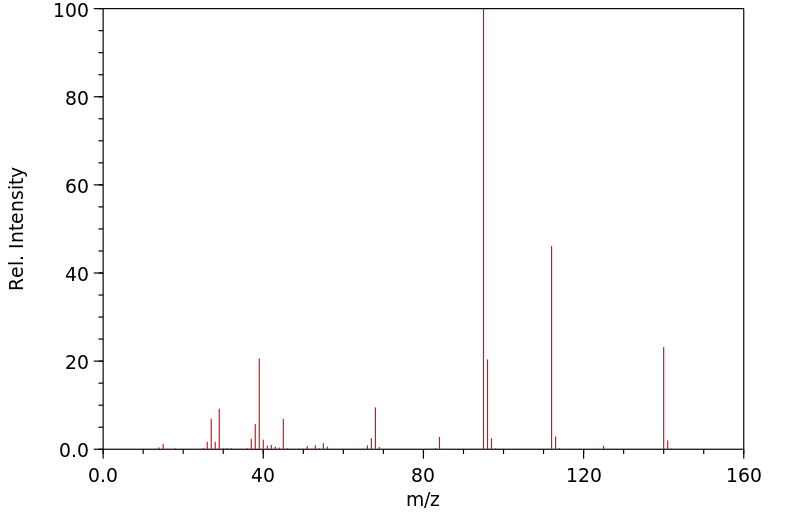
质谱MS
-
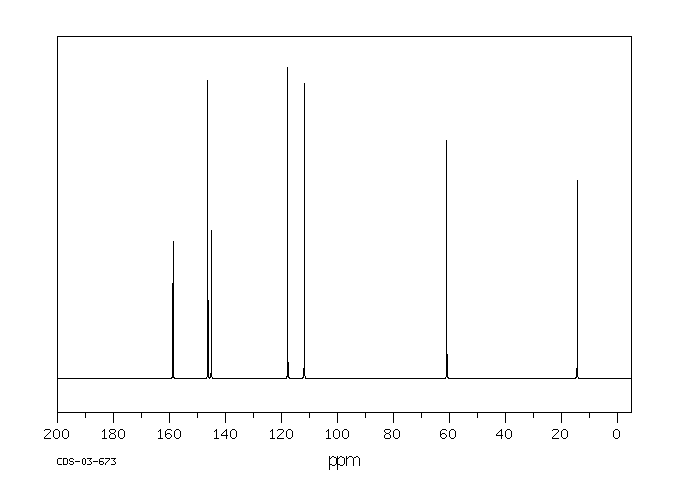
碳谱13CNMR
-
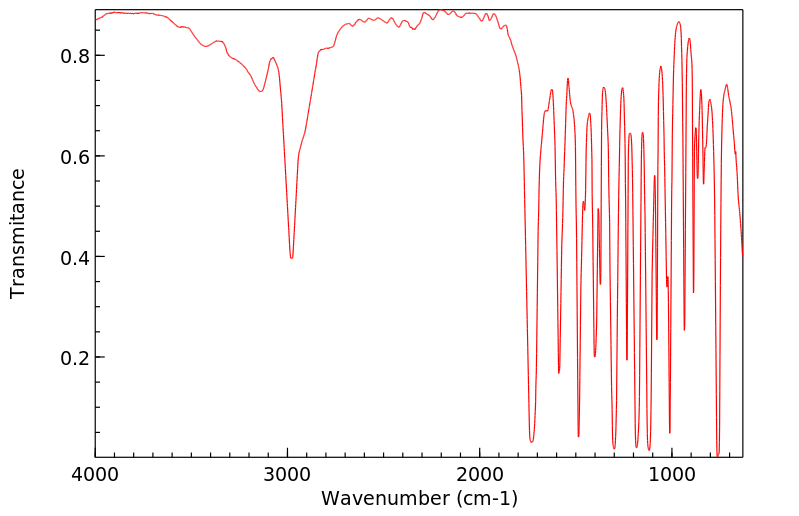
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
除草醚
锡烷,三丁基[(2-呋喃基羰基)氧代]-
醋糠硫胺
醋呋三嗪
酪氨酰-甘氨酰-色氨酰-蛋氨酰-门冬氨酰-苯基丙氨酰-甘氨酸
苯胺,N-[6-乙氧基-2,3-二(4-甲氧苯基)-4H-吡喃-4-亚基]-4-甲基-
糠酸(呋喃甲酸)
糠酸異戊酯
糠酸烯丙酯
碘化溴刚
硫代糠酸甲酯
硝基呋喃杂质
硝呋隆
硝呋醛肟标准品
硝呋达齐
硝呋美隆
硝呋维啶
硝呋立宗
硝呋甲醚
硝呋烯腙盐酸盐
硝呋烯腙
硝呋替莫
硝呋拉定
硝呋拉嗪
硝呋太尔杂质B
硝呋太尔杂质33
硝呋噻唑
硝呋吡醇
硝呋乙宗
盐酸呋喃它酮
盐酸呋喃他酮
疏呋那登
甲基7-[5-乙酰氨基-4-[(2-溴-4,6-二硝基苯基)偶氮]-2-甲氧苯基]-3-羰基-2,4,10-三氧杂-7-氮杂十一烷-11-酸酯
甲基5-溴-3-甲基-2-糠酸酯
甲基5-乙酰氨基-2-糠酸酯
甲基5-{[(氯乙酰基)氨基]甲基}-2-糠酸酯
甲基5-(甲氧基甲基)-2-甲基呋喃-3-羧酸酯
甲基5-(溴甲基)-4-(氯甲基)-2-糠酸酯
甲基5-(乙氧基甲基)-2-甲基-3-糠酸酯
甲基5-({[5-(三氟甲基)-2-吡啶基]硫代}甲基)-2-糠酸
甲基5-(4-甲酰基苯基)-2-糠酸酯
甲基5-(3-甲酰基苯基)-2-糠酸酯
甲基4-甲基-3-糠酸酯
甲基4-溴-5-甲基-2-糠酸酯
甲基4-乙酰基-5-甲基-2-糠酸酯
甲基4,6-二氯-3-(二乙基氨基)呋喃并[3,4-c]吡啶-1-羧酸酯
甲基3-羟基呋喃并[3,2-b]吡啶-2-羧酸酯
甲基3-甲酰基-2-糠酸酯
甲基3-氨基呋喃并[2,3-b]吡啶-2-羧酸酯
甲基3-氨基-5-(2-甲基-2-丙基)-2-糠酸酯