2-庚炔酸 | 1483-67-6
中文名称
2-庚炔酸
中文别名
2-庚缺酸
英文名称
hept-2-ynoic acid
英文别名
2-heptynoic acid
CAS
1483-67-6
化学式
C7H10O2
mdl
——
分子量
126.155
InChiKey
UEERQXQKEJPYBR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-3.32°C (estimate)
-
沸点:108-110°C 5mm
-
密度:0,98 g/cm3
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知危险反应,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:8
-
安全说明:S26,S36/37/39
-
危险类别码:R34
-
海关编码:2916190090
-
危险品运输编号:UN 3265
-
储存条件:请将贮藏器密封保存,并存放在阴凉干燥处。同时确保工作环境有良好的通风或排气设施。
SDS
2-Heptynoic Acid Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Heptynoic Acid
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1C
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
2-Heptynoic Acid
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Heptynoic Acid
Percent: >97.0%(T)
CAS Number: 1483-67-6
Chemical Formula: C7H10O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Comply with laws. Keep only in original container.
Packaging material:
2-Heptynoic Acid
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Very pale yellow - Reddish yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 141°C/3.2kPa
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.98
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
2-Heptynoic Acid
Section 12. ECOLOGICAL INFORMATION
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 3265
Proper shipping name: Corrosive liquid, acidic, organic, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Heptynoic Acid
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1C
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
2-Heptynoic Acid
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Heptynoic Acid
Percent: >97.0%(T)
CAS Number: 1483-67-6
Chemical Formula: C7H10O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Comply with laws. Keep only in original container.
Packaging material:
2-Heptynoic Acid
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Very pale yellow - Reddish yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 141°C/3.2kPa
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.98
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
2-Heptynoic Acid
Section 12. ECOLOGICAL INFORMATION
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 3265
Proper shipping name: Corrosive liquid, acidic, organic, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-庚炔酸乙酯 ethyl hept-2-ynoate 16930-95-3 C9H14O2 154.209 2-庚炔醛 hept-2-ynal 1846-67-9 C7H10O 110.156 2-庚炔-1-醇 hep-2-yn-1-ol 1002-36-4 C7H12O 112.172 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲基 2-庚炔酸盐 methyl 2-heptynoate 18937-78-5 C8H12O2 140.182 2-庚炔酸乙酯 ethyl hept-2-ynoate 16930-95-3 C9H14O2 154.209 —— isopropyl hept-2-ynoate 41519-21-5 C10H16O2 168.236 —— allyl hept-2-ynoate —— C10H14O2 166.22
反应信息
-
作为反应物:描述:参考文献:名称:TsNBr 2促进α,β-不饱和羧酸的脱羧溴化摘要:已经开发了使用N,N-二溴-对甲苯磺酰胺(TsNBr 2)的α,β-不饱和羧酸的脱羧溴化的快速方法。在乙腈中碳酸钾存在下,用TsNBr 2处理肉桂酸可生成相应的β-溴苯乙烯。(E的排他性在很短的时间内(5-15分钟)以立体选择性的方式观察到了)-β-溴苯乙烯。该方法进一步扩展为从相应的丙酸获得1-溴代炔烃。当在室温下在乙腈中以DBU为碱存在下进行反应时,观察到溴炔的瞬时形成。在温和的反应条件下,可以将各种各样的肉桂酸和丙酸分别转化为相应的β-溴代苯乙烯和1-溴代炔烃,且产率高至优异。DOI:10.1016/j.tetlet.2018.11.041
-
作为产物:描述:1-Chlorohept-2-ynylsulfanylbenzene 在 jones reagent 、 sodium carbonate 作用下, 以 丙酮 为溶剂, 反应 25.0h, 生成 2-庚炔酸参考文献:名称:从 2-乙酰苯硫醚合成 2-乙酰羧酸、1-亚磺酰基和 1-磺酰基-2-酮摘要:摘要 用磺酰氯在 1-位单氯化,然后甲醇分解将 2-炔基苯硫醚转化为 1-甲氧基-2-炔基苯硫醚。用铬酸氧化得到2-炔羧酸。用 Oxone® 和过氧化氢在乙酸中氧化 2-炔属苯硫醚,分别得到相应的亚砜和砜。二乙胺加成到三键产生烯胺,用盐酸水溶液将其水解成相应的 1-亚磺酰基和 1-磺酰基-2-酮。DOI:10.1080/00397919708005006
文献信息
-
Intramolecular ipso-arylative cyclization of aryl-alkynoates and N-arylpropiolamides with aryldiazonium salts through merged gold/visible light photoredox catalysis作者:Avinash H. Bansode、Samir R. Shaikh、Rajesh G. Gonnade、Nitin T. PatilDOI:10.1039/c7cc04010e日期:——A visible-light-promoted merged gold/photoredox catalyzed ipso-arylative cyclization has been reported. For instance, the reaction of aryl-alkynoates and N-arylpropiolamides with aryldiazonium salts in the presence of catalytic amounts of [(4-OCH3)C6H4]3PAuCl and Ru(bpy)3(PF6)2 under irradiation using a 32 W CFL bulb gave arylated spirocarbocycles in moderate to good yields.
-
Development of Gold-catalyzed [4+1] and [2+2+1]/[4+2] Annulations between Propiolate Derivatives and Isoxazoles作者:Rajkumar Lalji Sahani、Rai-Shung LiuDOI:10.1002/anie.201610665日期:2017.1.19new gold‐catalyzed annulations of isoxazoles with propiolates have been developed. Most isoxazoles follow an initial O attack on the alkyne to afford a [4+1] annulation product. This process results in a remarkable alkyne cleavage of initial propiolates. Unsubstituted isoxazoles proceed through an N attack step to yield formal [2+2+1]/[4+2] annulation products. These two annulation products arise initially
-
Platinum-Catalyzed Hydrosilylations of Internal Alkynes: Harnessing Substituent Effects to Achieve High Regioselectivity作者:Douglas A. Rooke、Eric M. FerreiraDOI:10.1002/anie.201108714日期:2012.3.26Rule of thumb: The high yielding title reaction is described with a focus on understanding the factors that govern the regioselectivity of the process (see scheme). Electronic, steric, and functional group properties all influence the selectivity, an understanding of which allows the selective formation of trisubstituted vinylsilanes, which are synthetically useful compounds for accessing stereodefined
-
[EN] FUMARATE ANALOGS AND USES THEREOF IN THE TREATMENT OF AN AUTOIMMUNE DISEASE OR AN INFLAMMATORY DISEASE<br/>[FR] ANALOGUES DE FUMARATE ET LEURS UTILISATIONS DANS LE TRAITEMENT D'UNE MALADIE AUTO-IMMUNE OU D'UNE MALADIE INFLAMMATOIRE申请人:RIGEL PHARMACEUTICALS INC公开号:WO2016022434A1公开(公告)日:2016-02-11Aspects of the present disclosure include compounds of formula (A) that find use for the treatment of a variety of autoimmune and inflammatory diseases and disorders. Embodiments of the present disclosure also relate to pharmaceutical compositions that include these compounds, methods of using these compounds in the treatment of various diseases and disorders, processes for preparing these compounds and intermediates useful in these processes. Formula (A).本公开涉及的方案包括式(A)的化合物,该化合物可用于治疗各种自身免疫性和炎症性疾病和疾病。本公开的实施例还涉及包括这些化合物的药物组合物,使用这些化合物治疗各种疾病和疾病的方法,制备这些化合物的过程以及在这些过程中有用的中间体。方案(A)。
-
Copper-Catalyzed Sequential Cyclization/Migration of Alkynyl Hydrazides for Construction of Ring-Expanded N–N Fused Pyrazolones作者:Keiji Konishi、Motohiro Yasui、Hitomi Okuhira、Norihiko Takeda、Masafumi UedaDOI:10.1021/acs.orglett.0c02378日期:2020.9.4copper-catalyzed sequential cyclization/migration reaction of alkynyl hydrazides for the synthesis of ring-expanded N–N fused pyrazolones was developed. Control experiments indicate that the copper–ligand complex plays an essential role in the reaction. This approach features a broad scope including some functional group tolerance as well as a nucleophilic addition/1,3-migration/formal 1,2-migration sequence. This
表征谱图
-
氢谱1HNMR
-
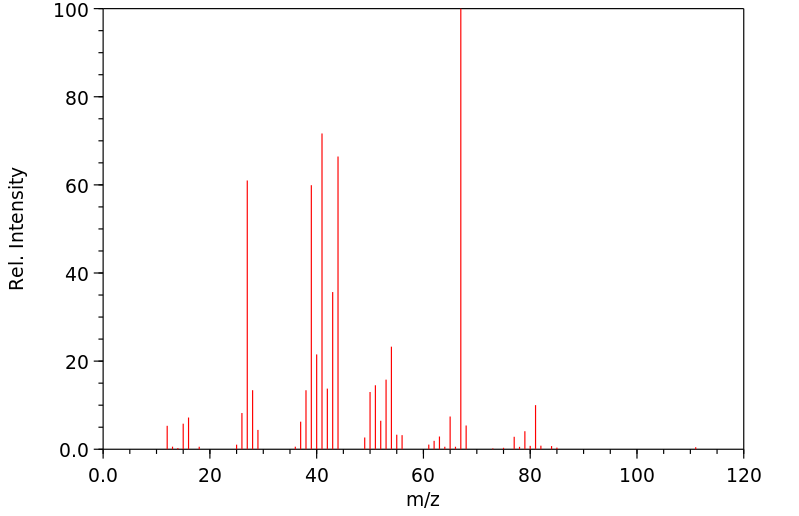
质谱MS
-
碳谱13CNMR
-
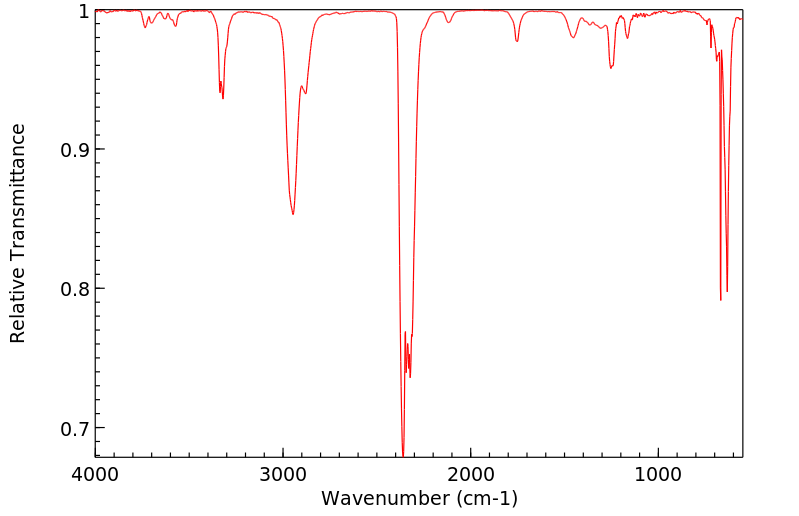
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯