甲基 2-庚炔酸盐 | 18937-78-5
中文名称
甲基 2-庚炔酸盐
中文别名
2-庚炔酸甲酯;甲基2-庚炔酸盐;2-庚炔甲酯
英文名称
methyl 2-heptynoate
英文别名
methyl hept-2-ynoate
CAS
18937-78-5
化学式
C8H12O2
mdl
——
分子量
140.182
InChiKey
IATZLNCRIIUXJM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.62
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2916190090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-庚炔酸 hept-2-ynoic acid 1483-67-6 C7H10O2 126.155
反应信息
-
作为反应物:参考文献:名称:三苯基氧化膦催化的共轭多不饱和酮的选择性 α,β-还原摘要:已经研究了使用三氯硅烷作为还原剂的三苯基氧化膦催化还原共轭多不饱和酮的范围。在所有研究的情况下,α,β-C=C 双键被选择性地还原为 C-C 单键,而所有其他可还原的官能团保持不变。该反应适用于多种共轭二烯酮、三烯酮和四烯酮。此外,还开发了串联一锅 Wittig/共轭还原反应序列,以直接从简单的构建单元生产 γ,δ-不饱和酮。在这些反应中,维蒂希反应的副产物作为还原反应的催化剂。然后将该策略用于合成天然存在的蛾信息素,以证明其在天然产物合成中的效用。DOI:10.1055/s-0037-1611537
-
作为产物:描述:参考文献:名称:Preparation, Properties and Derivatives of α-Acetylenic Acids1摘要:DOI:10.1021/ja01849a077
文献信息
-
Selective Synthesis of Dihydrophenanthridine and Phenanthridine Derivatives from the Cascade Reactions of <i>o</i>-Arylanilines with Alkynoates through C–H/N–H/C–C Bond Cleavage作者:Yuanshuang Xu、Caiyun Yu、Xinying Zhang、Xuesen FanDOI:10.1021/acs.joc.1c00256日期:2021.4.16unprecedented selective synthesis of dihydrophenanthridine and phenanthridine derivatives through the cascade reactions of 2-arylanilines with alkynoates is presented. Mechanistic studies showed that the formation of the dihydrophenanthridine scaffold involves an initial C(sp2)–H alkenylation of 2-arylaniline with alkynoate followed by an intramolecular aza-Michael addition. When this reaction is carried out
-
Nickel-catalyzed regioselective carbocyclization of ortho-halophenyl ketones with propiolates: an efficient route to disubstituted indenolsElectronic supplementary information (ESI) available: synthesis and characterization of compounds 3. See http://www.rsc.org/suppdata/cc/b2/b201473d/作者:Dinesh Kumar Rayabarapu、Chien-Hong ChengDOI:10.1039/b201473d日期:2002.4.19Carbocylization of o-halophenyl ketones with propiolates in the presence of Ni(dppe)Br2 and Zn powder in acetonitrile at 80 °C afforded the corresponding 2,3-disubstituted indenols.
-
Synthesis of Seven-membered Lactones via Nickel- and Zinc-Catalyzed Highly Regio- and Stereoselective Cyclization of 2-Iodobenzyl Alcohols with Propiolates作者:Dinesh Kumar Rayabarapu、Chien-Hong ChengDOI:10.1021/ja017390p日期:2002.5.1class of substituted seven-membered lactones 3 were conveniently synthesized via cyclization of o-iodobenzyl alcohol 1 (o-IC(6)H(4)CH(2)OH) with various propiolates 2 (RC triple bond CCOOMe) in the presence of Ni(dppe)Br(2) and Zn powder in acetonitrile at 80 degrees C. The catalytic reaction is highly regio- and stereoselective affording seven-membered lactones in moderate to good yields. This methodology
-
Novel Isomerically Pure Tetrasubstituted Allylboronates: Stereocontrolled Synthesis of α-Exomethylene γ-Lactones as Aldol-Like Adducts with a Stereogenic Quaternary Carbon Center作者:Jason W. J. Kennedy、Dennis G. HallDOI:10.1021/ja016391e日期:2002.2.11-alkoxycarbonyl vinylcopper(I) intermediates from the conjugate addition of organocuprates onto acetylenic esters were trapped with very high cis-addition selectivity with iodomethylboronic esters in the presence of HMPA. The resulting isomerically pure 3,3-disubstituted allylboronates react with aldehydes in a highly diastereo- and enantioselective manner, providing α-exomethylene γ-lactones with a stereogenic
-
Lewis Acid Catalyzed Allylboration: Discovery, Optimization, and Application to the Formation of Stereogenic Quaternary Carbon Centers作者:Jason W. J. Kennedy、Dennis G. HallDOI:10.1021/jo049773m日期:2004.6.1A full account of the development of the first catalytic manifold for the additions of allylboronates to aldehydes is described. The thermal additions (both diastereospecific and enantioselective) of 2-carboxyester 3,3-disubstituted allylboronates 1 to both aromatic and aliphatic aldehydes give biologically and synthetically important exo-methylene butyrolactones 2 containing a β-quaternary carbon
表征谱图
-
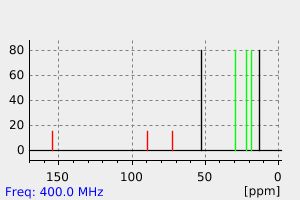
氢谱1HNMR
-
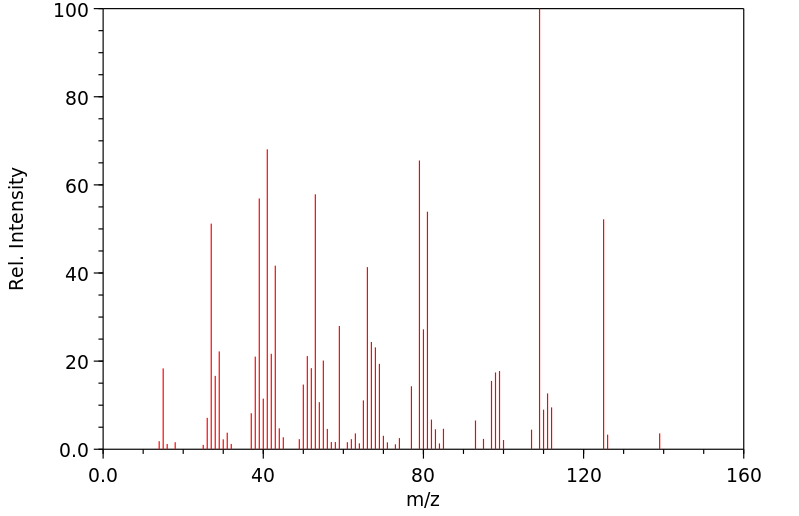
质谱MS
-
碳谱13CNMR
-
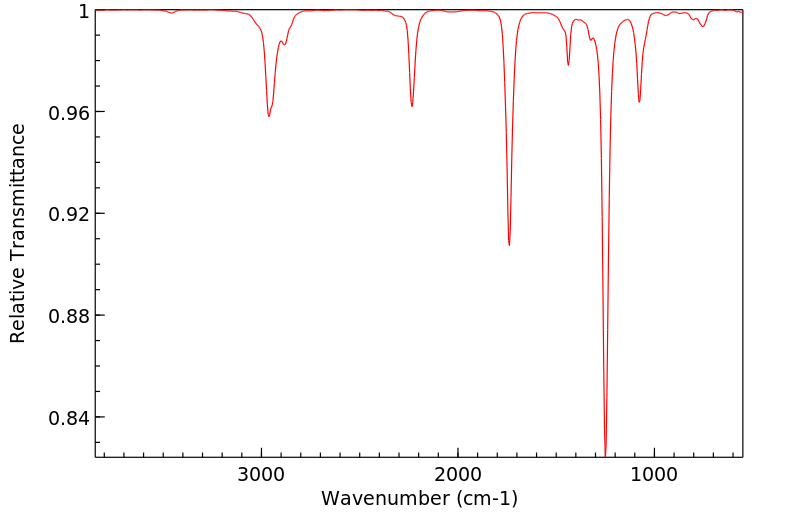
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯