2-溴-3,5,6-三甲基-2,5-环己二烯-1,4-二酮 | 7210-68-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3,5-三甲基-2,5-环己二烯-1,4-二酮 2,3,5-Trimethyl-1,4-benzoquinone 935-92-2 C9H10O2 150.177
反应信息
-
作为反应物:描述:参考文献:名称:一种三甲苯合成三甲基氢醌的方法摘要:本发明涉及一种三甲苯合成三甲基氢醌的方法,包括以下步骤:以偏三甲苯为原料,经过溴代反应、氧化反应和还原反应,最后制备得到三甲基氢醌;反应方程式为:本发明使用廉价的三甲苯为起始原料,工艺路线短,收率高,溴元素通过回收可以做到完全循环利用,是一条清洁的合成工艺,解决了现有工艺中间甲酚来源紧缺以及对二甲苯工艺中污染大的问题。公开号:CN106565423A
-
作为产物:描述:1,2,4-三甲基苯 在 potassium dichromate 、 硫酸 、 双氧水 、 碘 、 copper(II) sulfate 、 iron(II) sulfate 、 sodium bromide 作用下, 以 二氯甲烷 、 水 、 乙腈 为溶剂, 生成 2-溴-3,5,6-三甲基-2,5-环己二烯-1,4-二酮参考文献:名称:一种三甲苯合成三甲基氢醌的方法摘要:本发明涉及一种三甲苯合成三甲基氢醌的方法,包括以下步骤:以偏三甲苯为原料,经过溴代反应、氧化反应和还原反应,最后制备得到三甲基氢醌;反应方程式为:本发明使用廉价的三甲苯为起始原料,工艺路线短,收率高,溴元素通过回收可以做到完全循环利用,是一条清洁的合成工艺,解决了现有工艺中间甲酚来源紧缺以及对二甲苯工艺中污染大的问题。公开号:CN106565423A
文献信息
-
Solid state redox chemistry of hydroquinones and quinones作者:Jeroni Morey、José M. SaáDOI:10.1016/s0040-4020(01)80510-x日期:1993.1Solid state ceric ammonium nitrate (CAN) oxidation of hydroquinones to the corresponding quinones, gives best results when operating with ultrasonic irradiation. Nitrogen dioxide plays a key role in these “solid-solid” oxidations. The oxidation of hydroquinones to quinones can also be achieved in a unique “solid-solid-solid” reaction, i.e., by using a limited amount of CAN in the presence of a full
-
Acyl transfer accompanying the oxidation of monocarboxylic esters of hydroquinones作者:V. M. Clark、M. R. Eraut、D. W. HutchinsonDOI:10.1039/j39690000079日期:——Benzoyl transfer accompanies the oxidation of monobenzoates of hydroquinones. Oxidising agents used in the present investigation were ceric ion, thallic ion, N-bromosuccinimide, bromine, a high potential quinone, and lithium periodate. Evidence is presented that acylium intermediates are formed in the oxidations with ceric ion: thallic ion oxidations are catalysed by thallous ion, and acyl transfer in these
-
Oxidation Using Quaternary Ammonium Polyhalides. VIII. Oxidation of 1,4-Benzenediols with Benzyltrimethylammonium Tribromide作者:Shoji Kajigaeshi、Yukihiro Morikawa、Shizuo Fujisaki、Takaaki Kakinami、Keigo NishihiraDOI:10.1246/bcsj.64.336日期:1991.1The reaction of 1,4-benzenediols with 1.1 equiv of benzyltrimethylammonium tribromide in dichloromethane in the presence of aqueous sodium acetate at room temperature gave 2,5-cyclohexadiene-1,4-diones in good yields. On the other hand, the reaction of 1,4-benzenediols with a large excess of the reagent in aqueous acetic acid at 40–60°C gave polybromo-substituted 2,5-cyclohexadiene-1,4-diones in good1,4-苯二醇与1.1当量的苄基三甲基三溴化铵在二氯甲烷中在乙酸钠水溶液存在下在室温下反应以良好的产率得到2,5-环己二烯-1,4-二酮。另一方面,1,4-苯二醇与大量过量的试剂在 40-60°C 的乙酸水溶液中反应得到多溴取代的 2,5-环己二烯-1,4-二酮,收率良好。
-
Similarities and differences in the structures of 5-bromo-6-hydroxy-7,8-dimethylchroman-2-one and 6-hydroxy-7,8-dimethyl-5-nitrochroman-2-one作者:Shailesh K. Goswami、Lyall R. Hanton、C. John McAdam、Stephen C. Moratti、Jim SimpsonDOI:10.1107/s0108270113005325日期:2013.4.15
The title compounds, C11H11BrO3, (I), and C11H11NO5, (II), respectively, are derivatives of 6-hydroxy-5,7,8-trimethylchroman-2-one substituted at the 5-position by a Br atom in (I) and by a nitro group in (II). The pyranone rings in both molecules adopt half-chair conformations, and intramolecular O—H...Br [in (I)] and O—H...Onitro[in (II)] hydrogen bonds affect the dispositions of the hydroxy groups. Classical intermolecular O—H...O hydrogen bonds are found in both molecules but play quite dissimilar roles in the crystal structures. In (I), O—H...O hydrogen bonds form zigzag
C (9) chains of molecules along thea axis. Because of the tetragonal symmetry, similar chains also form alongb . In (II), however, similar contacts involving an O atom of the nitro group form inversion dimers and generateR 22(12) rings. These also result in a close intermolecular O...O contact of 2.686 (4) Å. For (I), four additional C—H...O hydrogen bonds combine with π–π stacking interactions between the benzene rings to build an extensive three-dimensional network with molecules stacked along thec axis. The packing in (II) is much simpler and centres on the inversion dimers formed through O—H...O contacts. These dimers are stacked through additional C—H...O hydrogen bonds, and further weak C—H...O interactions generate a three-dimensional network of dimer stacks.标题化合物 C11H11BrO3(I)和 C11H11NO5(II)分别是 6-羟基-5,7,8-三甲基苯并吡喃-2-酮的衍生物,(I) 的 5 位被一个 Br 原子取代,(II) 的 5 位被一个硝基取代。这两个分子中的吡喃酮环都采用半周构象,分子内的 O-H...Br(在 (I) 中)和 O-H...Onitro(在 (II) 中)氢键影响羟基的排列。两种分子中都存在典型的分子间 O-H...O 氢键,但在晶体结构中的作用却大相径庭。在 (I)中,O-H...O 氢键沿轴向形成人字形 C(9)分子链。由于呈四方对称,沿 b 轴也会形成类似的分子链。但在(II)中,涉及硝基的一个 O 原子的类似接触会形成反转二聚体并生成 R22(12)环。这也导致分子间的 O...O 接触达到 2.686 (4) Å。对于 (I),另外四个 C-H...O氢键与苯环之间的π-π堆积相互作用相结合,构建了一个广泛的三维网络,分子沿轴向堆积。(II)中的堆积要简单得多,主要是通过 O-H...O 接触形成的反向二聚体。这些二聚体通过额外的 C-H...O 氢键堆叠,进一步的微弱 C-H...O 相互作用产生了二聚体堆叠的三维网络。 -
Dimerization Reactions of 2-Bromo-3,5,6-trimethyl-1,4-benzoquinone作者:Shuhei Azuma、Motohiro Ota、Akito Ishida、Katsuhiro Isozaki、Hikaru Takaya、Masaharu Nakamura、Takahiro Sasamori、Norihiro Tokitoh、Kouji Kuramochi、Kazunori TsubakiDOI:10.1246/cl.130781日期:2013.12.5The dimerization reactions of 2-bromo-3,5,6-trimethyl-1,4-benzoquinone have been examined. 2,2′-Dimeric benzoquinone was prepared via a Stille-type homocoupling, whereas an oxepin was formed by the treatment of the title quinone with hexamethylditin, CuI, and (±)-BINAP in DMF. Two dimerization reactions involving a hetero Diels–Alder reaction between quinones and quinone methides were also observed.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
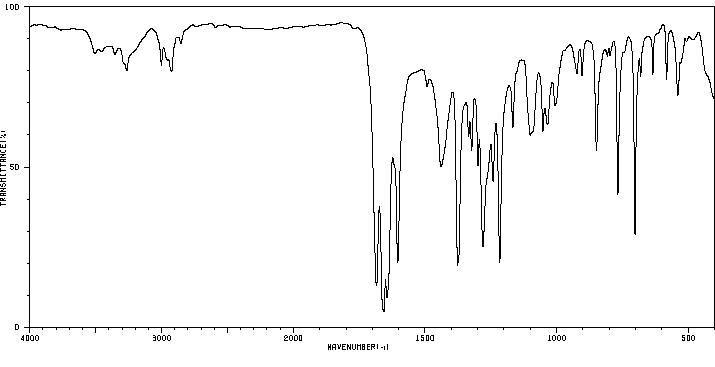
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息