2-溴-4,6-二-叔-丁基苯酚 | 20834-61-1
中文名称
2-溴-4,6-二-叔-丁基苯酚
中文别名
2-溴-4,6-二叔丁基苯酚
英文名称
2-Bromo-4,6-di-tert-butylphenol
英文别名
2-bromo-4,6-ditert-butylphenol
CAS
20834-61-1
化学式
C14H21BrO
mdl
MFCD00051597
分子量
285.224
InChiKey
DIWZVAHZEOFSLS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56 °C
-
沸点:141-142 °C
-
密度:1.201±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):5.6
-
重原子数:16
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.571
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:T
-
安全说明:S22,S26,S36/37/39,S45
-
危险类别码:R23/24/25,R36/37/38
-
海关编码:2908199090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:2811
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封,并将其放入一个紧密封装的容器中。建议存放在阴凉、干燥的地方。
SDS
| Name: | 2-Bromo-4 6-di-tert-butylphenol 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 20834-61-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 20834-61-1 | 2-Bromo-4,6-di-tert-butylphenol | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 20834-61-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: tan
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 50 - 53 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H21BrO
Molecular Weight: 285
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, acid chlorides, bases.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 20834-61-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Bromo-4,6-di-tert-butylphenol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 20834-61-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 20834-61-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 20834-61-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二叔丁基苯酚 2,4-di-tert-Butylphenol 96-76-4 C14H22O 206.328 2,4,6-三叔丁基苯酚 2,4,6-tri-tert-butylphenoxol 732-26-3 C18H30O 262.436 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-4,6-di-tert-butylanisole 217819-14-2 C15H23BrO 299.251 —— 1-bromo-3,5-di-tert-butyl-2-ethoxybenzene 292166-97-3 C16H25BrO 313.278 —— 1-bromo-3,5-di-tert-butyl-2-propyloxybenzene 245435-08-9 C17H27BrO 327.305 —— 1-bromo-3,5-di-tert-butyl-2-(methoxymethoxy)benzene 191478-95-2 C16H25BrO2 329.277 —— 2-bromo-4,6-di-tert-butylphenyl trimethylsilyl ether 5920-84-3 C17H29BrOSi 357.406 —— 2,4-di-tert-butyl-6-bromophenyl benzyl ether 245434-17-7 C21H27BrO 375.349 —— 3,5-di-tert-butyl-2-hydroxybenzonitrile 95091-86-4 C15H21NO 231.338 3,5-二叔丁基邻苯二酚 3,5-Di-tert-butylcatechol 1020-31-1 C14H22O2 222.327 (2,4-二叔丁基苯氧基)三甲基硅烷 (2,4-di-tert-butylphenoxy)trimethylsilane 53925-65-8 C17H30OSi 278.51 3,5-二叔丁基水杨酸 3,5-ditertbutyl salicylic acid 19715-19-6 C15H22O3 250.338
反应信息
-
作为反应物:描述:2-溴-4,6-二-叔-丁基苯酚 在 四(三苯基膦)钯 、 正丁基锂 、 sodium carbonate 作用下, 以 甲醇 、 乙醚 、 正己烷 、 甲苯 为溶剂, 反应 90.0h, 生成 6,6′-di(3,5-di-tert-butyl-2-hydroxybenzene)-2,2′-bipyridine参考文献:名称:铁席夫碱络合物电催化还原CO 2生成甲酸酯摘要:6,6'-二(3,5-二叔丁基-2-羟基苯)-2,2'-联吡啶(Fe(tbu dhbpy报道了在电化学还原条件下的)Cl)。在二氧化碳的存在下(CO 2在无水的条件下)N,N-二甲基甲酰胺(DMF),该复合物介导2E - CO两个当量的还原性歧化2到一氧化碳(CO)和碳酸(CO 3 2-)。在CO 2下添加苯酚(PhOH)作为质子源时饱和时,观察到催化电流;受控电势电解实验的产物分析表明,大多数产物为甲酸盐(68±4%法拉第效率),H 2为次要产物(30±10%法拉第效率)和最低限度的CO(1.1±0.3%法拉第效率)。根据从循环伏安法和红外光谱电化学(IR-SEC)获得的数据,中间体铁羰基物质中CO的释放极其缓慢,并且竞争性降解,从而限制了该催化剂的活性和寿命。机理研究还表明,与Fe配位的酚盐部分在还原状态下对质子化敏感,表明合作的侧挂质子相互作用可能参与了CO 2的还原。DOI:10.1021/acs.inorgchem.7b02955
-
作为产物:描述:2,4-二叔丁基苯酚 在 高氯酸 、 双氧水 、 trans-dioxidomolybdenium(VI)-meso-tetra(p-chlorophenul)porphirin 、 potassium bromide 作用下, 以 水 为溶剂, 以99 %的产率得到2-溴-4,6-二-叔-丁基苯酚参考文献:名称:反式二氧化钼(VI)卟啉及其模拟氧化溴化的催化活性摘要:合成了反式二氧化钼(VI)-内消旋四苯基卟啉和反式二氧化钼(VI)-内消旋四(对氯苯基)卟啉,并用作模拟苯酚及其衍生物氧化溴化的仿生卤过氧化物酶的催化剂。DOI:10.1002/ejic.202400001
-
作为试剂:参考文献:名称:遷移金属化合物、オレフィン重合用触媒およびオレフィン系重合体の製造方法摘要:提供含有新型过渡金属化合物和优良的烯烃聚合活性的烯烃聚合用催化剂。采用以下化合物(D)代表的过渡金属化合物,以及包含该过渡金属化合物,最好包括有机金属化合物、有机铝氧化合物以及与上述过渡金属化合物反应形成离子对的化合物的烯烃系聚合体用催化剂。【选择图】无公开号:JP2015193612A
文献信息
-
Chiral 2-(2-hydroxyaryl)alcohols (HAROLs) with a 1,4-diol scaffold as a new family of ligands and organocatalysts作者:Ömer Dilek、Mustafa A. Tezeren、Tahir Tilki、Erkan ErtürkDOI:10.1016/j.tet.2017.11.054日期:2018.1Efficient and modular syntheses of chiral 2-(2-hydroxyaryl)alcohols (HAROLs), novel 1,4-diols carrying one phenolic and one alcohol hydroxyl group, have been developed which led to generation of a small library of structurally diverse HAROLs in enantiomerically pure form. Of the different HAROLs examined, a HAROL based on the indan backbone exhibited the highest activity and enantioselectivity in the
-
[EN] COMPOSITIONS AND METHODS FOR IMMUNE MODULATION AND TREATMENT OF CANCER<br/>[FR] COMPOSITIONS ET MÉTHODES DE MODULATION IMMUNITAIRE ET DE TRAITEMENT DU CANCER申请人:UNIV MICHIGAN STATE公开号:WO2020150668A1公开(公告)日:2020-07-23The disclosure relates to rexinoids, including compounds of the Formula (I) and (II) or a pharmaceutically acceptable salt, polymorph, prodrug, solvate or clathrate thereof. These rexinoids are useful for increasing PD-L1 in vivo, for treatment of cancer, and for inhibiting the onset of cancer.该披露涉及雷克西酮,包括式(I)和(II)的化合物或其药用可接受的盐、多型体、前药、溶剂合物或包合物。这些雷克西酮对于体内增加PD-L1,在癌症治疗中以及抑制癌症发生方面是有用的。
-
Imidazolin-2-iminato ligand supported titanium(IV) aryloxo complexes – Syntheses and structures作者:Kishor Naktode、Suman Das、Hari Pada Nayek、Tarun K. PandaDOI:10.1016/j.ica.2016.11.010日期:2017.2and tris- aryloxo titanium (IV) complexes supported by imidazolin-2-iminato ligands. The reaction of titanium imidazolin-2-iminato complexes [(ImRN)Ti(NMe2)3] [R = tBu (tert-butyl) (1a), Mes (mesityl) (1b) and Dipp (2,6-diisopropylphenyl) (1c)] with various substituted phenols in 1:1 M ratio in toluene afforded the corresponding mixed mono-aryloxo imidazolin-2-iminato titanium (IV) complexes of general摘要我们报道了由咪唑啉-2-亚氨基配体支撑的一系列单,双和三芳基氧钛(IV)配合物的合成和结构。咪唑啉-2-亚氨基钛钛配合物[[(ImRN)Ti(NMe2)3] [R = tBu(叔丁基)(1a),Mes(甲磺酰基)(1b)和Dipp(2,6-二异丙基苯基)( [1c)],在甲苯中以1:1 M的比例用各种取代的苯酚提供相应的通式[(ImRN)Ti(NMe2)2(OAr')]的混合单芳氧基咪唑啉-2-亚氨基钛(IV)配合物[ (R = Mes,Ar'= 2,6-tBu2C6H3(2b),2,4-tBu2-4-Me-C6H2(3b),2-Br-4,6-tBu2- (4b),R =浸入,Ar'= 2,4-tBu2-4-Me- (3c),2-Br-4,6-tBu2- (4c)]。与2 M取代酚的类似反应产生相应的双(芳基氧代)咪唑啉-2-亚氨基钛(IV)络合物[(ImtBuN)Ti(NMe2)(OAr')2]
-
Thallium in organic synthesis—XVI作者:A. McKillop、B.P. Swann、E.C. TaylorDOI:10.1016/s0040-4020(01)93043-1日期:1970.1Treatment of 2,6-disubstituted-4-t-butylphenols with thallium(III) trifluoroacetate in either trifluoroacetic acid or carbon tetrachloride as solvent results in loss of the 4-t-butyl substituent as isobutene and formation in high yield of the corresponding 2,6-disubstituted p-quinones. Possible mechanisms for this novel reaction, which probably proceeds via the intermediacy of a hydroquinone or hydroquinone
-
Bicyclic aromatic compounds and pharmaceutical/cosmetic compositions comprised thereof申请人:Galderma Research & Development S.N.C.公开号:US06632963B1公开(公告)日:2003-10-14Novel pharmaceutically/cosmetically-active bicyclic aromatic compounds have the structural formula (I): in which Ar1 is a radical having one of the structural formulae (a)-(c): Ar2 is a radical having one of the following formulae (d)-(h): and X is a radical having one of the following formulae (i)-(l): and are useful for the treatment of a wide variety of disease states, whether human or veterinary, for example dermatological, rheumatic, respiratory, cardiovascular and ophthalmological disorders, as well as for the treatment of mammalian skin and hair conditions/disorders.新型具有药用/化妆活性的双环芳香化合物具有结构式(I): 其中Ar1是具有以下结构式之一的基团(a)-(c): Ar2是具有以下结构式之一的基团(d)-(h): X是具有以下结构式之一的基团(i)-(l): 这些化合物可用于治疗各种疾病状态,无论是人类还是兽医,例如皮肤病、风湿病、呼吸系统疾病、心血管疾病和眼科疾病,以及治疗哺乳动物的皮肤和毛发状况/疾病。
表征谱图
-
氢谱1HNMR
-
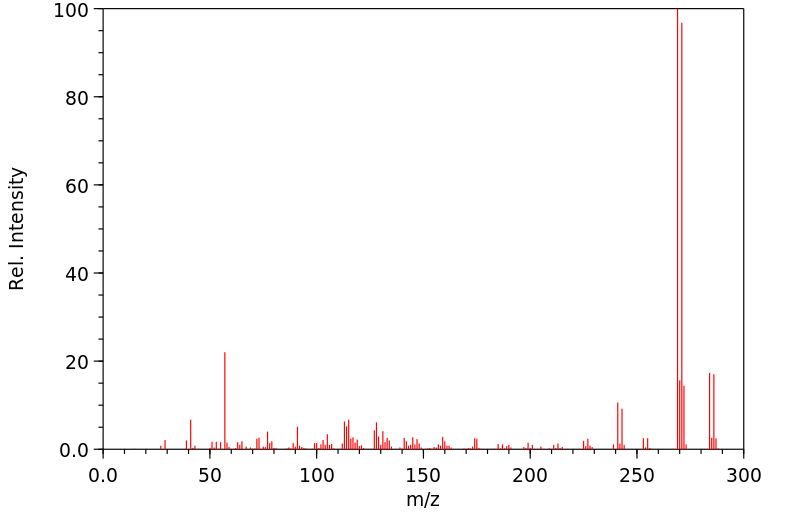
质谱MS
-
碳谱13CNMR
-
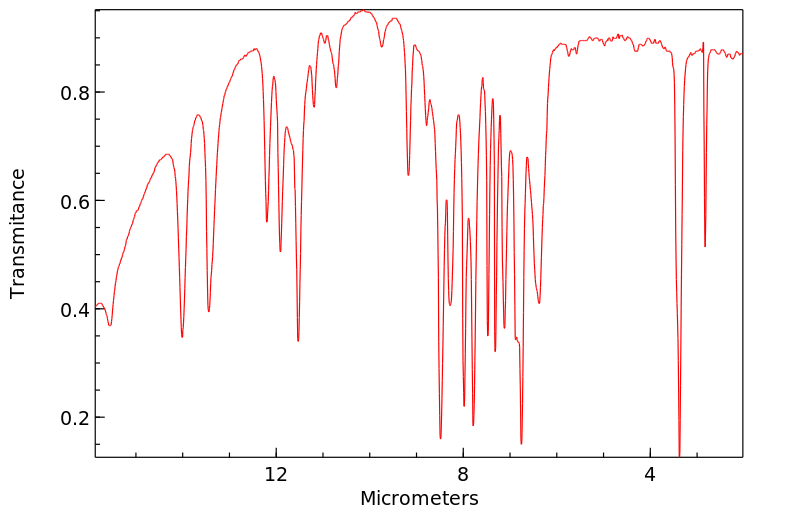
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫