3,4,5-三氯水杨苯胺 | 642-84-2
中文名称
3,4,5-三氯水杨苯胺
中文别名
3',4',5-三氯水扬苯胺;3',4',5-三氯水杨苯胺;5-氯-N-(3,4-二氯苯基)-2-羟基苯甲酰胺
英文名称
3',4',5-trichlorosalicylanilide
英文别名
5-chloro-N-(3,4-dichlorophenyl)-2-hydroxybenzamide
CAS
642-84-2
化学式
C13H8Cl3NO2
mdl
MFCD00059622
分子量
316.571
InChiKey
RSJBLPJKXGNMFW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:247°C
-
沸点:388.3±42.0 °C(Predicted)
-
密度:1.5338 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):5.2
-
重原子数:19
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:49.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2924299090
-
储存条件:室温
SDS
3',4',5-Trichlorosalicylanilide
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3',4',5-Trichlorosalicylanilide
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3',4',5-Trichlorosalicylanilide
Percent: >98.0%(T)
CAS Number: 642-84-2
Chemical Formula: C13H8Cl3NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
3',4',5-Trichlorosalicylanilide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
3',4',5-Trichlorosalicylanilide
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3',4',5-Trichlorosalicylanilide
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3',4',5-Trichlorosalicylanilide
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3',4',5-Trichlorosalicylanilide
Percent: >98.0%(T)
CAS Number: 642-84-2
Chemical Formula: C13H8Cl3NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
3',4',5-Trichlorosalicylanilide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
3',4',5-Trichlorosalicylanilide
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3',4',5-Trichlorosalicylanilide
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl ethylcarbamate 1219818-69-5 C16H13Cl3N2O3 387.65 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl butylcarbamate 1219818-70-8 C18H17Cl3N2O3 415.704 —— [4-Chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl] benzoate 1422904-45-7 C20H12Cl3NO3 420.679 —— 4-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl (3-chloropropyl)carbamate —— C17H14Cl4N2O3 436.122 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl pentylcarbamate 1219818-71-9 C19H19Cl3N2O3 429.73 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl hexylcarbamate 1219818-72-0 C20H21Cl3N2O3 443.757 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl heptylcarbamate 1219818-73-1 C21H23Cl3N2O3 457.784 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl undecylcarbamate 1219818-77-5 C25H31Cl3N2O3 513.892 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl nonylcarbamate 1219818-75-3 C23H27Cl3N2O3 485.838 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl octylcarbamate 1219818-74-2 C22H25Cl3N2O3 471.811 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl decylcarbamate 1219818-76-4 C24H29Cl3N2O3 499.865 —— 4-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl diethyl phosphate 1552270-31-1 C17H17Cl3NO5P 452.658 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 4-(trifluoromethyl)benzoate 1403607-23-7 C21H11Cl3F3NO3 488.677 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-(benzyloxycarbonylamino)acetate 1121627-48-2 C23H17Cl3N2O5 507.757 —— 4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl pyrazine-2-carboxylate 1426258-43-6 C18H10Cl3N3O3 422.655 —— (S)-4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-(benzyloxycarbonylamino)propanoate 1169776-66-2 C24H19Cl3N2O5 521.784 —— (R)-4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-(benzyloxycarbonylamino)propanoate 1169776-68-4 C24H19Cl3N2O5 521.784 —— (S)-4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-acetamido-3-phenylpropanoate 1261296-10-9 C24H19Cl3N2O4 505.785 —— (S)-4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-(benzyloxycarbonylamino)-3-methylbutanoate 1169776-70-8 C26H23Cl3N2O5 549.838 —— (R)-4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-(benzyloxycarbonylamino)-3-methylbutanoate 1169776-72-0 C26H23Cl3N2O5 549.838 —— (S)-4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-(benzyloxycarbonylamino)-3-phenylpropanoate 1169776-74-2 C30H23Cl3N2O5 597.882 —— (R)-4-chloro-2-(3,4-dichlorophenylcarbamoyl)phenyl 2-(benzyloxycarbonylamino)-3-phenylpropanoate 1169776-76-4 C30H23Cl3N2O5 597.882 —— 5-chloro-N-[(2S)-1-(3,4-dichloroanilino)-1-oxo-3-phenylpropan-2-yl]-2-hydroxybenzamide 1227477-06-6 C22H17Cl3N2O3 463.748 —— 5-chloro-N-[(2R)-1-(3,4-dichloroanilino)-1-oxo-3-phenylpropan-2-yl]-2-hydroxybenzamide 1227477-07-7 C22H17Cl3N2O3 463.748 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Investigating Spectrum of Biological Activity of 4- and 5-Chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides摘要:在本研究中,描述了一系列二十二种5-氯-2-羟基-N-[2-(芳胺基)-1-烷基-2-氧乙基]苯甲酰胺和十种4-氯-2-羟基-N-[2-(芳胺基)-1-烷基-2-氧乙基]苯甲酰胺。使用RP-HPLC分析这些化合物以确定其亲脂性。对合成的化合物进行了针对分枝杆菌、细菌和真菌株的初步体外筛选。还评估了它们对菠菜(Spinacia oleracea L.)叶绿体中光合电子传递(PET)的抑制活性。这些化合物的生物活性与标准药物异烟肼、氟康唑、青霉素G或环丙沙星相当或更高。对于所有化合物,讨论了其亲脂性与化学结构之间的关系以及其构效关系。DOI:10.3390/molecules16032414
-
作为产物:描述:参考文献:名称:基于喹喔啉的外排泵抑制剂可恢复耐药非结核分枝杆菌的药物敏感性摘要:非结核分枝杆菌 (NTM) 包括几种普遍存在的环境局部细菌,这些细菌可能导致严重的人类疾病。NTM 相关肺部感染主要影响患有潜在呼吸道疾病或慢性疾病的个体和免疫抑制患者。模拟分枝杆菌和脓肿分枝杆菌是导致免疫功能正常和免疫功能低下个体肺部疾病的两种 NTM。在这项研究中,从意大利一家医院收治的两名患者身上分离出两种 NTM 菌株,分别被鉴定为M. simiae和M. abscessus. 测试了两种 NTM 对不同抗生素的药物敏感性。为了恢复药物敏感性,设计、合成了一系列新的 2-aryl-3-phenoxymethyl-quinoxaline 衍生物 (QX),并将其作为外排泵抑制剂 (EPI) 对上述 NTM 的两种临床分离株进行研究,评估 EPI 如何可以影响药物的最小抑制浓度值,从而影响活性。在临床菌株中追踪的不同耐药水平被 EPI 降低,并且在一些情况下,易感性完全恢复。QX 还可DOI:10.1002/ardp.202100492
文献信息
-
Kappa agonist compounds and pharmaceutical formulations thereof申请人:——公开号:US20030144272A1公开(公告)日:2003-07-31Compounds having kappa opioid agonist activity, compositions containing them and method of using them as analgesics are provided. The compounds of formulae I, II, IIA, III, IIIA, IIIB, IIIB-i, IV and IVA have the structure: 1 2 wherein R 1 , R 2 , R 3 , R 4 ; and X, X 4 , X 5 , X 7 , X 9 ; Y, Z and n are as described in the specification.提供具有kappa阿片受体激动剂活性的化合物,含有这些化合物的组合物以及使用它们作为镇痛剂的方法。 具有以下结构的化合物I、II、IIA、III、IIIA、IIIB、IIIB-i、IV和IVA: 1 2 其中 R 1 ,R 2 ,R 3 ,R 4 ;和 X,X 4 ,X 5 ,X 7 ,X 9 ; Y,Z和n如规范中所述。
-
Carboxamide and amino derivatives and methods of their use申请人:Dolle E. Roland公开号:US20050113294A1公开(公告)日:2005-05-26Carboxamide and amino derivatives, pharmaceutical compositions containing these compounds, and methods for their pharmaceutical use are disclosed. In certain embodiments, the carboxamide derivatives are ligands of the δ opioid receptor and are useful, inter alia, for treating and/or preventing pain, anxiety, gastrointestinal disorders, and other δ opioid receptor-mediated conditions.
-
2-Hydroxy-N-phenylbenzamides and Their Esters Inhibit Acetylcholinesterase and Butyrylcholinesterase作者:Martin Krátký、Šárka Štěpánková、Neto-Honorius Houngbedji、Rudolf Vosátka、Katarína Vorčáková、Jarmila VinšováDOI:10.3390/biom9110698日期:——The development of novel inhibitors of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) represents a viable approach to alleviate Alzheimer's disease. Thirty-six halogenated 2-hydroxy-N-phenylbenzamides (salicylanilides) with various substitution patterns and their esters with phosphorus-based acids were synthesized in yields of 72% to 92% and characterized. They were evaluated for in新型乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)抑制剂的开发代表了减轻阿尔茨海默氏病的可行方法。合成了36种具有各种取代方式的卤代2-羟基-N-苯基苯甲酰胺(水杨酰苯胺)及其与磷基酸的酯,收率72%至92%,并进行了表征。使用改良的Ellman分光光度法评估了它们对电鳗AChE和马血清BuChE的体外抑制作用。苯甲酰胺在33.1至85.8 µM的窄浓度范围内表现出对AChE的中等抑制作用,IC50值较高。BuChE的IC50值较高(53.5-228.4 µM)。大多数衍生物比BuChE更有效地抑制AChE,并且与rivastigmine(一种用于治疗阿尔茨海默氏病的成熟胆碱酯酶抑制剂)相当或优于。磷基酯尤其具有5-氯-2-[4-(三氟甲基)苯基]氨基甲酰基}苯基二乙基亚磷酸酯5c优越的BuChE活性(IC50 = 2.4 µM)。该衍生物也是BuChE的最选择性抑制剂。它引起了两
-
New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains作者:Aleš Imramovský、Jarmila Vinšová、Juana Monreal Férriz、Rafael Doležal、Josef Jampílek、Jarmila Kaustová、Filip KuncDOI:10.1016/j.bmc.2009.04.008日期:2009.5better bio-availability and as more efficient transport forms through the mycobacterial cell membranes due to the higher lipophilicity. The experimental and calculated lipophilicity, stability, antituberculotic activity, cytotoxicity as well as the quantitative structure–activity relationships (QSARs) explored by the Intelligent Problem Solver (IPS) in Trajan Neural Network Simulator 6.0 are presented
-
Fused bicyclic carboxamide derivatives and methods of their use申请人:Dolle E. Roland公开号:US20050054630A1公开(公告)日:2005-03-10Fused bicyclic carboxamide derivatives are disclosed. Pharmaceutical compositions containing the compounds and methods for their use are also disclosed.揭示了融合的双环羧酰胺衍生物。还公开了含有这些化合物的药物组合物以及它们的使用方法。
表征谱图
-
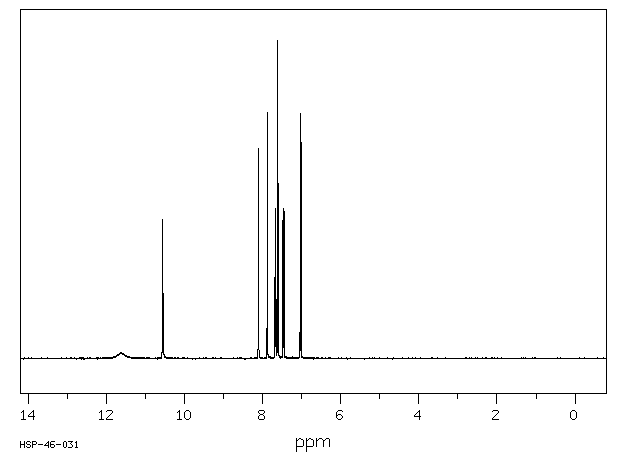
氢谱1HNMR
-
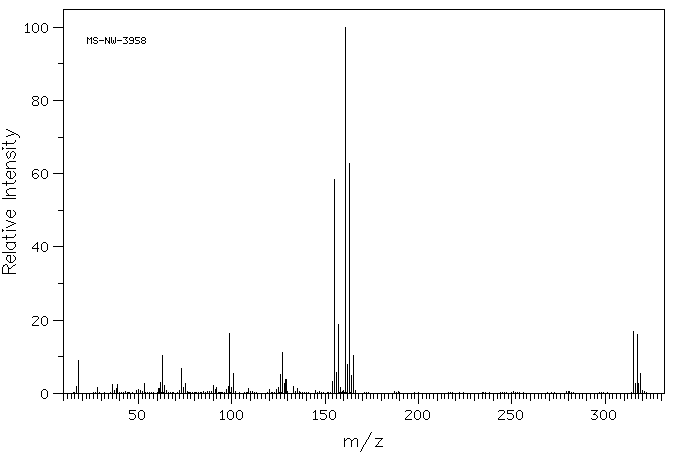
质谱MS
-
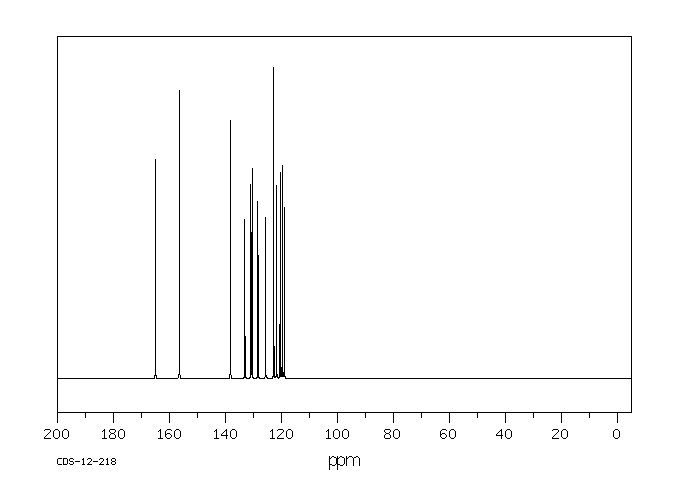
碳谱13CNMR
-
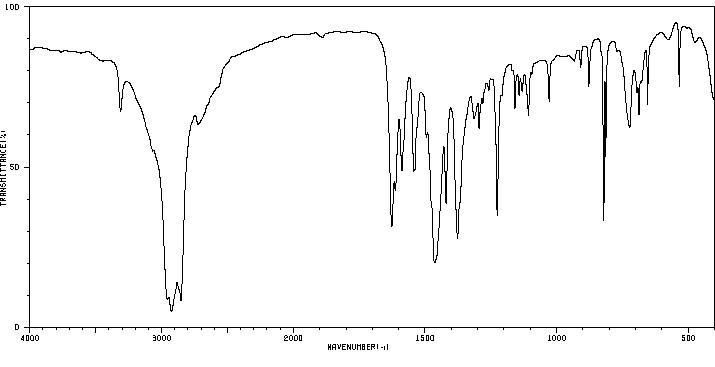
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫