3-(4-硝基苯基)-2-丙炔-1-醇 | 61266-32-8
中文名称
3-(4-硝基苯基)-2-丙炔-1-醇
中文别名
——
英文名称
3-(4-nitrophenyl)prop-2-yn-1-ol
英文别名
3-(4-nitrophenyl)-2-propyn-1-ol
CAS
61266-32-8
化学式
C9H7NO3
mdl
MFCD00168844
分子量
177.159
InChiKey
IVTAMNNBGCKOBI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.111
-
拓扑面积:66
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
海关编码:2906299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-乙炔基-4-硝基苯 (4-Nitrophenyl)acetylene 937-31-5 C8H5NO2 147.133 —— 2-((3-(4-nitrophenyl)prop-2-yn-1-yl)oxy)tetrahydro-2H-pyran 61266-31-7 C14H15NO4 261.277 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— O-(3-(4-nitrophenyl)-2-propynyl)hydroxylamine 1287768-76-6 C9H8N2O3 192.174 —— 1-(3-methoxyprop-1-yn-1-yl)-4-nitrobenzene —— C10H9NO3 191.186 1-硝基-4-(1-丙炔-1-基)苯 1-nitro-4-(prop-1-yn-1-yl)benzene 28289-83-0 C9H7NO2 161.16 1-(3-溴丙-1-炔基)-4-硝基苯 1-(3-bromoprop-1-yn-1-yl)-4-nitrobenzene 61266-34-0 C9H6BrNO2 240.056 —— 3-(4-nitrophenyl)propiolonitrile 62501-58-0 C9H4N2O2 172.143 —— 3-(4-nitrophenyl)prop-2-yn-1-amine 1211534-92-7 C9H8N2O2 176.175 (4-硝基苯基)炔丙醛 3-(4-nitrophenyl)prop-2-ynal 35487-34-4 C9H5NO3 175.144 1-(3-氯丙-1-炔基)-4-硝基苯 1-(3-chloroprop-1-ynyl)-4-nitrobenzene 41412-79-7 C9H6ClNO2 195.605 1-乙炔基-4-硝基苯 (4-Nitrophenyl)acetylene 937-31-5 C8H5NO2 147.133 —— methylthio (4-(4-nitro)phenyl)butadiyne 1446871-08-4 C11H7NO2S 217.248 —— 3-(4-nitrophenyl)prop-2-yn-1-yl methanesulfonate 1613190-39-8 C10H9NO5S 255.251 —— isopropyl (3-(4-nitrophenyl)-2-propynyl)oxycarbamate 1287768-92-6 C13H14N2O5 278.265 —— benzyl (3-(4-nitrophenyl)-2-propynyl)oxycarbamate 1287768-77-7 C17H14N2O5 326.309 —— p-Nitro-phenylallen 38319-12-9 C9H7NO2 161.16 3-(4-硝基苯基)-1-丙醇 3-(4-nitrophenyl)propan-1-ol 20716-25-0 C9H11NO3 181.191 4-硝基肉桂醇 3-(4-nitrophenyl)propyl-2-en-1-ol 1504-63-8 C9H9NO3 179.175 - 1
- 2
反应信息
-
作为反应物:描述:3-(4-硝基苯基)-2-丙炔-1-醇 在 Lindlar's catalyst titanium(IV) isopropylate 、 叔丁基过氧化氢 、 sodium azide 、 L-(+)-酒石酸二乙酯 、 水 、 氢气 、 silica gel 、 三苯基膦 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 168.0h, 生成 氯霉素参考文献:名称:Asymmetric synthesis of chloramphenicol摘要:采用(2S,3R)-4-硝基苯基缩水甘油醇路线,描述了氯霉素的对映选择性合成。DOI:10.1039/c39920000859
-
作为产物:参考文献:名称:Garratt-Braverman环化中的选择性:实验和计算研究摘要:合成了具有不同性质的芳环的双炔丙基砜。在基本条件下,这些砜异构化为双亚烯砜,在两个替代的Garratt-Braverman(GB)环化途径之间创造了竞争格局。观察到的产物分布排除了任何离子中间体的参与,并通过更多的亲核基团支持了富电子的芳环的更大参与的双自由基机理。基于DFT的计算支持双自由基机理以及观察到的选择性。DOI:10.1021/ol102861j
文献信息
-
Discovery of Potent Human Glutaminyl Cyclase Inhibitors as Anti-Alzheimer’s Agents Based on Rational Design作者:Van-Hai Hoang、Phuong-Thao Tran、Minghua Cui、Van T. H. Ngo、Jihyae Ann、Jongmi Park、Jiyoun Lee、Kwanghyun Choi、Hanyang Cho、Hee Kim、Hee-Jin Ha、Hyun-Seok Hong、Sun Choi、Young-Ho Kim、Jeewoo LeeDOI:10.1021/acs.jmedchem.7b00098日期:2017.3.23proposed binding mode of the preferred substrate, Aβ3E−42. An in vitro structure–activity relationship study identified several excellent QC inhibitors demonstrating 5- to 40-fold increases in potency compared to a known QC inhibitor. When tested in mouse models of AD, compound 212 significantly reduced the brain concentrations of pyroform Aβ and total Aβ and restored cognitive functions. This potent Aβ-lowering谷氨酰胺基环化酶(QC)通过产生β淀粉样肽(pGlu-Aβ)的N末端焦谷氨酸与毒性淀粉样蛋白斑块的形成有关,因此可能参与了阿尔茨海默氏病(AD)的发病机理。我们基于优选底物Aβ3E-42的拟议结合模式设计了谷氨酰环化酶(QC)抑制剂库。一项体外结构-活性关系研究确定了几种出色的QC抑制剂,与已知的QC抑制剂相比,其效能提高了5至40倍。在AD的小鼠模型中测试时,化合物212显着降低了焦状Aβ和总Aβ的大脑浓度,并恢复了认知功能。这种强大的Aβ降低作用是通过将一个额外的结合区并入我们先前建立的药效团模型中而实现的,从而导致在QC结合位点与Glu327的羧酸酯基团发生强相互作用。我们的研究为设计新型QC抑制剂作为AD的潜在治疗方法提供了有用的见识。
-
An improved general method for palladium catalyzed alkenylations and alkynylations of aryl halides under microwave conditions作者:Andrea Togninelli、Harsukh Gevariya、Maddalena Alongi、Maurizio BottaDOI:10.1016/j.tetlet.2007.05.086日期:2007.7Palladium catalyzed facile method for alkenylation and alkynylation of arylhalides in good to excellent yield under microwave condition is reported.报道了在微波条件下钯催化的芳基卤的烯基化和炔基化的简便方法。
-
Catalytic Access to Functionalized Allylic <i>gem</i> ‐Difluorides via Fluorinative Meyer–Schuster‐Like Rearrangement作者:Lihao Liao、Rui An、Huimin Li、Yang Xu、Jin‐Ji Wu、Xiaodan ZhaoDOI:10.1002/anie.202003897日期:2020.6.26functionalized allylic gem‐difluorides via catalytic fluorinative Meyer–Schuster‐like rearrangement is disclosed. This transformation proceeded with readily accessible propargylic fluorides, and low‐cost B–F reagents and electrophilic reagents by sulfide catalysis. A series of iodinated, brominated, and trifluoromethylthiolated allylic gem‐difluorides that were difficult to access by other methods were
-
Sonogashira Reaction of Aryl and Heteroaryl Halides with Terminal Alkynes Catalyzed by a Highly Efficient and Recyclable Nanosized MCM-41 Anchored Palladium Bipyridyl Complex作者:Bo-Nan Lin、Shao-Hsien Huang、Wei-Yi Wu、Chung-Yuan Mou、Fu-Yu TsaiDOI:10.3390/molecules15129157日期:——A heterogeneous catalyst, nanosized MCM-41-Pd, was used to catalyze the Sonogashira coupling of aryl and heteroaryl halides with terminal alkynes in the presence of CuI and triphenylphosphine. The coupling products were obtained in high yields using low Pd loadings to 0.01 mol%, and the nanosized MCM-41-Pd catalyst was recovered by centrifugation of the reaction solution and re-used in further runs without significant loss of reactivity.
-
Highly <i>Z</i>-selective synthesis of 1,3-oxathiol-2-ylidenes and 4-methylene-oxazolidine-2-thiones <i>via</i> atom-specific 5-<i>exo-dig</i> cyclization of propargyl alcohol with isothiocyanate作者:S. Antony Savarimuthu、D. G. Leo Prakash、S. Augustine Thomas、Thirumanavelan Gandhi、Mrinal K. BeraDOI:10.1039/d0ob00083c日期:——internal propargyl alcohol to produce (Z)-1,3-oxathiol-2-ylidenes and (Z)-N-(Z)-4-ethylidene-1,3-oxathiolan-2-ylidenes from secondary and primary propargyl alcohols, respectively. The formation of high Z-selectivity in the imine motif and alkene is the highlight of this new method as multiple selectivities over C[double bond, length as m-dash]N and C[double bond, length as m-dash]C in a single system are syntheticallyDBU介导的异硫氰酸酯和炔丙醇的5-exo-dig环化反应可形成有价值的杂环化合物。发现异硫氰酸酯的亲核性的不同模式(S-选择性或N-选择性)取决于炔丙醇的取代模式。对末端炔丙醇和异硫氰酸酯进行N-亲核攻击,得到3-取代的4-亚甲基恶唑烷-2-硫酮。相反,观察到内部炔丙醇的独家S亲核环化反应产生(Z)-1,3-氧杂硫醇-2-亚烷基和(Z)-N-(Z)-4-亚乙基-1,3-氧杂硫杂环戊烷-分别来自仲和伯炔丙醇的2-亚烷基。在亚胺基序和烯烃中高Z选择性的形成是这种新方法的亮点,因为在C [双键,在单个系统中,如m-N和C双键的长度,如m-C的长度在合成上是非常具有挑战性的。亚胺和烯烃中的Z-选择性可以分别归因于电子和空间因素。
表征谱图
-
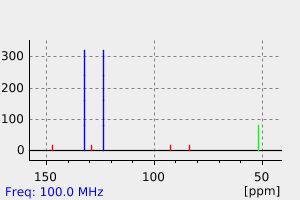
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫