3-乙酰基-4-羟基喹啉-2(1h)-酮 | 26138-64-7
中文名称
3-乙酰基-4-羟基喹啉-2(1h)-酮
中文别名
——
英文名称
4-hydroxy-3-acetyl-1H-quinoline-2-one
英文别名
3-acetyl-4-hydroxyquinolin-2(1H)-one;3-Acetyl-4-hydroxycarbostyril;3-acetyl-4-hydroxy-2(1H)-quinolinone;3-Acetyl-4-hydroxy-2-quinolone;3-acetyl-4-hydroxy-1H-quinolin-2-one
CAS
26138-64-7
化学式
C11H9NO3
mdl
——
分子量
203.197
InChiKey
UTOJPZBVJLHYGO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2933790090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-喹啉二醇 4-Hydroxy-2-quinolone 86-95-3 C9H7NO2 161.16 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-Hydroxy-3-(3-phenylacryloyl)quinolin-2(1h)-one 671758-27-3 C18H13NO3 291.306 —— 4-hydroxy-3-[3-(4-methoxyphenyl)-1-oxo-2-propen-1-yl]-2(1Η)-quinolinone 163232-52-8 C19H15NO4 321.332 —— 4-hydroxy-3-[(2E)-3-(4-methoxyphenyl)prop-2-enoyl]quinolin-2(1H)-one —— C19H15NO4 321.332
反应信息
-
作为反应物:描述:3-乙酰基-4-羟基喹啉-2(1h)-酮 在 sodium acetate 、 一水合肼 作用下, 以 乙醇 为溶剂, 反应 9.0h, 以75%的产率得到(Z)-3-(1-hydrazonoethyl)-4-hydroxyquinoline-2(1H)-one参考文献:名称:Synthesis, antioxidant and toxicological study of novel pyrimido quinoline derivatives from 4-hydroxy-3-acyl quinolin-2-one摘要:A series of novel pyrimido and other fused quinoline derivatives like 4-methyl pyrimido [5,4-c]quinoline-2,5(1H,6H)-dione (4a), 4-methyl-2-thioxo-1,2-dihydropyrimido [5,4-c]quinoline-5(6H)-one (4b), 2-amino-4-methyl-1,2-dihydropyrimido [5,4-c]quinolin-5(6H)-one (4c), 3-methylisoxazolo [4,5-c]quinolin-4(5H)-one (4d), 3-methyl-1H-pyrazolo [4,3-c]quinoline-4(5H)-one (5e), 5-methyl-1H-[1,2,4] triazepino [6,5-c]quinoline-2,6(3H,7H)-dione (5f), 5-methyl-2-thioxo-2,3-dihydro-1H-[1,2,4]triazepino [6,5-c]quinolin-6(7H)one (5g) were synthesized regioselectively from 4-hydroxy-3-acyl quinolin-2-one 3. They were screened for their in vitro antioxidant activities against radical scavenging capacity using DPPH, Trolox equivalent antioxidant capacity (TEAC), total antioxidant activity by FRAP, superoxide radical (O-2(degrees-)) scavenging activity, metal chelating activity and nitric oxide scavenging activity. Among the compounds screened, 4c and 5g exhibited significant antioxidant activities. (C) 2010 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2010.09.018
-
作为产物:参考文献:名称:Synthesis of 3-Substituted 4-Hydroxyquinolin-2-ones via C-Acylation Reactions of Active Methylene Compounds with Functionalized 3,1-Benzoxazin-4-ones摘要:DOI:10.3987/com-99-8508
文献信息
-
Substituted quinolinones. 18. 3-Acetyl-4-methylthioquinolin-2(1H)-one as useful synthon intermediate for synthesis of some new quinolinones作者:Mohamed M. Hassan、Elham S. Othman、Mohamed AbassDOI:10.1007/s11164-012-0678-7日期:2013.3reactions with some binucleophiles, hydrazine, hydroxylamine, urea, thiourea, semicarbazide, and thiosemicarbazide, furnishing some known five, six, and seven heterocyclic annellated quinolines. All the new compounds have been characterized using different spectral and analytical tools.
-
Robust synthesis of linear and angular furoquinolines using Rap–Stoermer reaction作者:Devadoss Karthik Kumar、Rayappan Rajkumar、Subramaniam Parameswaran RajendranDOI:10.1007/s10593-016-1885-8日期:2016.5Rap–Stoermer reaction by conventional as well as microwave method that furnished an enhanced yield. Initially, we synthesized linear furo[2,3-b]quinolines from 3-acetyl-6-chloro-4-phenyl-1H-quinolin-2-one and three different α-halocarbonyl compounds: chloroacetophenone, ethyl chloroacetate, and chloroacetamide. The scope of the methodology was further extended to the synthesis of angular furo[3,2-c]quinolines
-
Substituted 1, 4-thiazepine and analogs as activators of caspases and inducers of apoptosis and the use thereof申请人:Cytovia, Inc.公开号:US20020010169A1公开(公告)日:2002-01-24The present invention is directed to substituted 1,4-thiazepine and analogs thereof, represented by the general Formula I: 1 wherein the dashed lines, A 1 , A 2 , A 3 , X 1 and R 1 are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of capases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
-
Reactions of 2-methyl-3,1-benzoxazin-4-one with active methylene compounds: a new route to 3-substituted 4-hydroxyquinolin-2(1H)-ones作者:Anastasia Detsi、Vassilios Bardakos、John Markopoulos、Olga Igglessi-MarkopoulouDOI:10.1039/p19960002909日期:——A new route to 3-substituted 4-hydroxyquinolin-2(1H)-ones, compounds of great biological importance, is described. The C-acylation of active methylene compounds 2 with the 2-methyl-3,1-benzoxazin-4-one 1, under basic conditions, leads to the formation of the new products 3–10, which have been isolated and characterized. Cyclization of the above intermediates furnishes the 3-substituted 4-hydroxyquinolin-2(1H)-ones. Spectral data and physical characteristics for all compounds are reported.
-
Novel quinolinone–pyrazoline hybrids: synthesis and evaluation of antioxidant and lipoxygenase inhibitory activity作者:Ioanna Kostopoulou、Antonia Diassakou、Eleni Kavetsou、Eftichia Kritsi、Panagiotis Zoumpoulakis、Eleni Pontiki、Dimitra Hadjipavlou-Litina、Anastasia DetsiDOI:10.1007/s11030-020-10045-x日期:2021.5pyrazoline analogues (9a-9o). All the synthesized analogues were in vitro evaluated in terms of their antioxidant and soybean lipoxygenase (LOX) inhibitory activity. Among all the pyrazoline derivatives, compounds 9b and 9m were found to possess the best combined activity, whereas 9b analogue exhibited the most potent LOX inhibitory activity, with IC50 value 10 μM. The in silico docking results revealed that本项目致力于研究几种喹啉酮-查尔酮和喹啉酮-吡唑啉杂化物的构效关系,以期发现有前景的抗氧化剂和抗炎剂。为了实现这一目标,通过羟醛缩合反应合成了四种生物活性杂化喹啉酮-查尔酮化合物(8a-8d),然后进行化学修饰,形成十五种新的吡唑啉类似物(9a-9o)。所有合成的类似物均在体外评估其抗氧化和大豆脂氧合酶(LOX)抑制活性。在所有吡唑啉衍生物中,化合物9b和9m被发现具有最佳的综合活性,而9b类似物表现出最强的LOX抑制活性,IC50值为10 μM。计算机对接结果显示,合成的吡唑啉类似物 9b 显示出较高的 AutoDock Vina 评分(- 10.3 kcal/mol),而所有测试的衍生物均表现出与酶的变构相互作用。
表征谱图
-
氢谱1HNMR
-
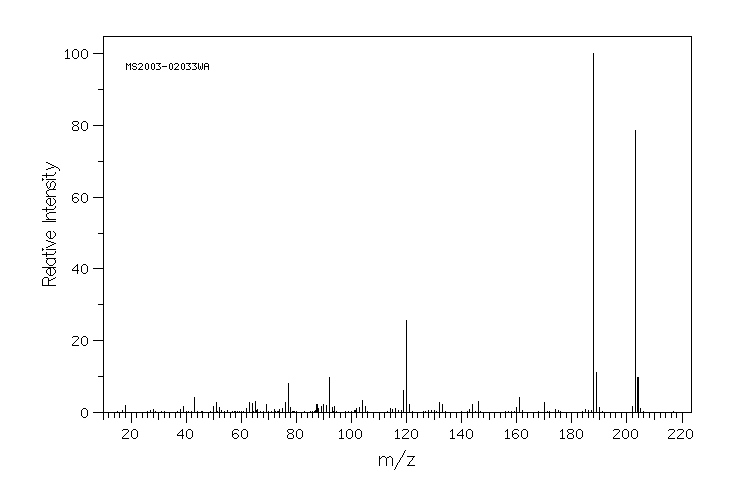
质谱MS
-
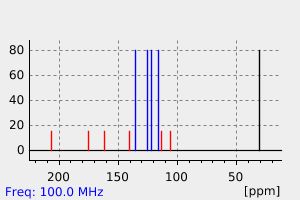
碳谱13CNMR
-
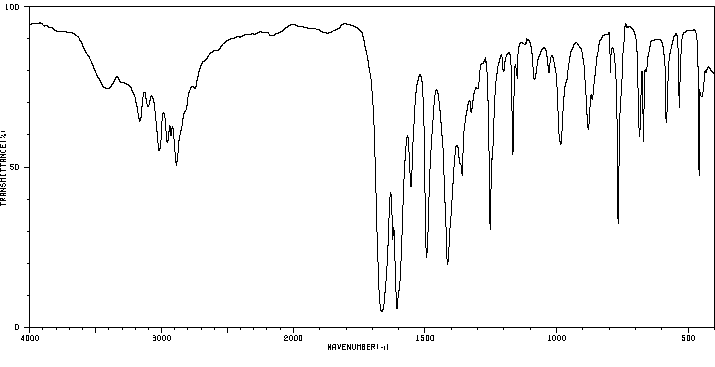
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43