3-庚炔 | 2586-89-2
中文名称
3-庚炔
中文别名
——
英文名称
hept-3-yne
英文别名
3-heptyne;Hept-3-in;3-Heptin
CAS
2586-89-2
化学式
C7H12
mdl
MFCD00039960
分子量
96.1723
InChiKey
KLYHSJRCIZOUHE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-130.5°C
-
沸点:105-106 °C(lit.)
-
密度:0.741 g/mL at 25 °C(lit.)
-
闪点:2 °F
-
保留指数:744
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品标志:F,Xi
-
安全说明:S16,S26,S36/37/39
-
危险类别码:R11
-
WGK Germany:3
-
海关编码:2901299090
-
危险品运输编号:UN 3295 3/PG 1
-
储存条件:密封在阴凉干燥的环境中。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 3-Heptyne
CAS-No. : 2586-89-2
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Flammable liquids (Category 2)
Skin irritation (Category 2)
Eye irritation (Category 2)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Highly flammable. Irritating to eyes, respiratory system and skin.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Danger
Hazard statement(s)
H225 Highly flammable liquid and vapour.
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautionary statement(s)
P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R11 Highly flammable.
R36/37/38 Irritating to eyes, respiratory system and skin.
S-phrase(s)
S16 Keep away from sources of ignition - No smoking.
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C7H12
Molecular Weight : 96,17 g/mol
Component Concentration
Hept-3-yne
CAS-No. 2586-89-2 -
EC-No. 219-967-7
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
For small (incipient) fires, use media such as "alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large quantities (flooding) of water applied as a mist or
spray; solid streams of water may be ineffective. Cool all affected containers with flooding quantities of
water.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
Use water spray to cool unopened containers.
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation.
Remove all sources of ignition. Evacuate personnel to safe areas. Beware of vapours accumulating to
form explosive concentrations. Vapours can accumulate in low areas.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Contain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and
place in container for disposal according to local regulations (see section 13).
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Keep away from sources of ignition - No smoking.Take measures to prevent the build up of electrostatic
charge.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Impervious clothing., Flame retardant antistatic protective clothing, The type of protective
equipment must be selected according to the concentration and amount of the dangerous
substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
Colour: colourless
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 105 - 106 °C - lit.
boiling range
g) Flash point -16 °C - closed cup
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 0,741 g/cm3 at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
Heat, flames and sparks. Extremes of temperature and direct sunlight.
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Causes respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin
May be harmful if absorbed through skin. Causes skin irritation.
Eyes Causes serious eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Burn in a chemical incinerator equipped with an afterburner and scrubber but exert extra care in igniting
as this material is highly flammable. Offer surplus and non-recyclable solutions to a licensed disposal
company. Contact a licensed professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: 3295 IMDG: 3295 IATA: 3295
UN proper shipping name
ADR/RID: HYDROCARBONS, LIQUID, N.O.S.
IMDG: HYDROCARBONS, LIQUID, N.O.S.
IATA: Hydrocarbons, liquid, n.o.s.
Transport hazard class(es)
ADR/RID: 3 IMDG: 3 IATA: 3
Packaging group
ADR/RID: II IMDG: II IATA: II
Environmental hazards
ADR/RID: no IMDG Marine Pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-辛炔 4-Octyne 1942-45-6 C8H14 110.199 3-己炔 hex-3-yne 928-49-4 C6H10 82.1454 2-戊炔 2-Pentyne 627-21-4 C5H8 68.1185 1-戊炔 1-Pentyne 627-19-0 C5H8 68.1185 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-辛炔 4-Octyne 1942-45-6 C8H14 110.199 3-己炔 hex-3-yne 928-49-4 C6H10 82.1454 1-庚烯-3-炔 hept-1-en-3-yne 2384-73-8 C7H10 94.1564 —— 2,2-dimethyl-3-heptyne 29022-29-5 C9H16 124.226 1-辛烯-3-炔 oct-1-en-3-yne 17679-92-4 C8H12 108.183
反应信息
-
作为反应物:参考文献:名称:Behal, Annales de Chimie (Cachan, France), 1888, vol. <6> 15, p. 421摘要:DOI:
-
作为产物:描述:参考文献:名称:Photochemistry of organic iodides. III. Photolytic formation of isomeric vinyl radicals from cis- and trans-vinyl iodides摘要:DOI:10.1021/jo01276a001
文献信息
-
Selectivity controllable divergent synthesis of α,β-unsaturated amides and maleimides from alkynes and nitroarenes via palladium-catalyzed carbonylation作者:Jin-Bao Peng、Hui-Qing Geng、Fu-Peng Wu、Da Li、Xiao-Feng WuDOI:10.1016/j.jcat.2019.05.034日期:2019.7A tunable procedure on palladium-catalyzed carbonylative divergent synthesis of α,β-unsaturated amides and maleimides from symmetrical internal alkynes and nitroarenes has been developed. By using Pd(acac)2/dppp/4-ClBSA/DMF and Pd(TFA)2/DPEphos/PTSA/H2O/toluene as the catalytic systems, a range of multi-substituted maleimides and α,β-unsaturated amides were selectively prepared in moderate to good
-
Nickel-Catalyzed Asymmetric Reductive [3 + 2] Annulation of <i>o</i>-Haloaromatic β-Alkenyl Ketones with Alkynes via Alkene Isomerization: Enantioselective Synthesis of 1-Alkenyl 1<i>H</i>-Inden-1-ols作者:Ya-Fei Han、Yang Li、Xuan-Hui Ouyang、Ming Hu、Ze Tan、Jin-Heng LiDOI:10.1021/acscatal.1c02613日期:2021.8.20nickel-catalyzed asymmetric reductive [3 + 2] annulation of o-haloaromatic β-alkenyl ketones with alkynes through alkyne dicarbofunctionalization and alkene isomerization cascades is disclosed, enabling the formation of highly enantiomerically enriched 1-alkenyl 1H-inden-1-ols that possess an important stereogenic quaternary carbon and an active alkene unit. This reaction shows notable features of a broad substrate
-
Regiocontrolled Cu<sup>I</sup>-Catalyzed Borylation of Propargylic-Functionalized Internal Alkynes作者:Abraham L. Moure、Ramón Gómez Arrayás、Diego J. Cárdenas、Inés Alonso、Juan C. CarreteroDOI:10.1021/ja300627s日期:2012.5.2orbitalic influence from the propargylic group, matched with ligand and substrate size effects, as key factors involved in the high β-selectivity. The vinylboronates allowed the stereoselective synthesis of trisubstituted olefins, while allylic substitution of the SO(2)Py group without affecting the boronate group provided access to formal hydroboration products of unbiased dialkylalkynes.
-
Polylithiumorganic compounds作者:Adalbert Maercker、Andrea Tatai、Burkhard Grebe、Ulrich GirreserDOI:10.1016/s0022-328x(01)01195-0日期:2002.1observed. The metalation products 5–7 show follow-up reactions like 1,3-H shift to the corresponding 1-lithio-1-alkynes 8 and subsequent metalation to the dilithioalkynes 9. Additionally, lithium hydride elimination and ring-chain rearrangement (for 5c) are observed. 1,2-Hexadiene (3b) can be brought to reaction with lithium metal in the apolar solvent pentane, here the follow-up reactions are much slower研究了丙烯(3a)与烷基取代的丙烯1,2-己二烯(3b),环丙烯(3c)和亚乙烯基环丙烷(3d)与锂金属的反应,以获得2,3-二硫代烯烃4a - d。这些二硫代烯烃4a - d在极性溶剂(如THF)中具有很高的反应性,并作为强碱,可以观察到起始丙二烯3a - d或该溶剂的金属化或足够酸性的中间体(如8 a - d)。金属制品5 – 7显示后续反应,如1,3-H转移到相应的1-lithio-1-炔烃8和随后的金属化到dilithioalkynes 9。此外,观察到氢化锂消除和环链重排(对于5c)。1,2-己二烯(3b)可以在非极性溶剂戊烷中与锂金属反应,由于4b的不溶性,因此后续反应要慢得多。在所有情况下,通过用简单的亲电试剂淬灭,形成区域异构体和立体异构体产物的复杂混合物阻碍了对反应途径的阐明。
-
Reusable Gold(I) Catalysts with Unique Regioselectivity for Intermolecular Hydroamination of Alkynes作者:Antonio Leyva、Avelino CormaDOI:10.1002/adsc.200900491日期:2009.11Two gold(I) phosphine complexes bearing the low-coordinating bis(trifluoromethanesulfonyl)imidate ligand, namely AuSPhosNTf2 and AuPPh3NTf2, are active catalysts for the regioselective intermolecular hydroamination of both internal and terminal alkynes under mild reaction conditions. The catalysts show a regioselectivity based on electronic rather than steric factors, which allow the preferential synthesis
表征谱图
-
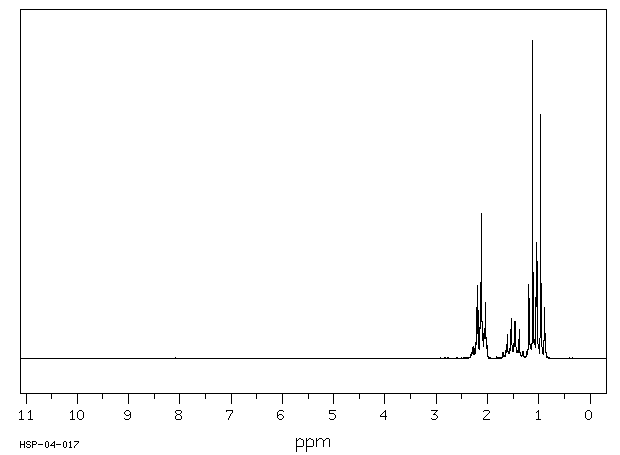
氢谱1HNMR
-
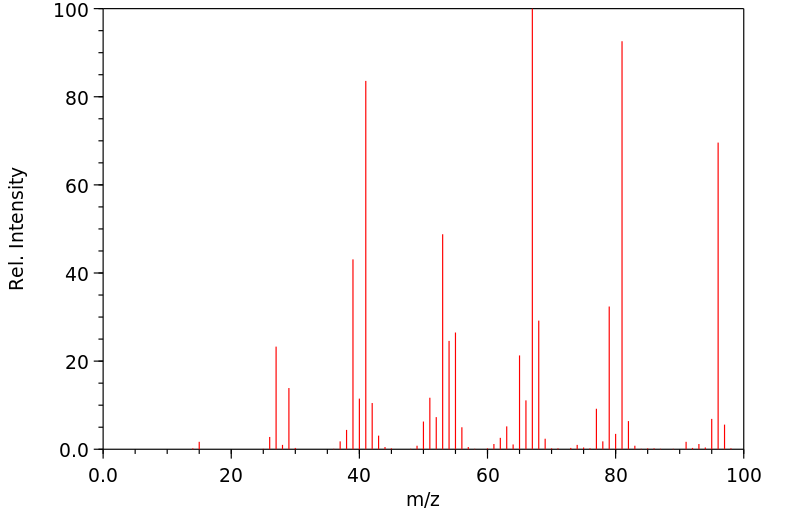
质谱MS
-
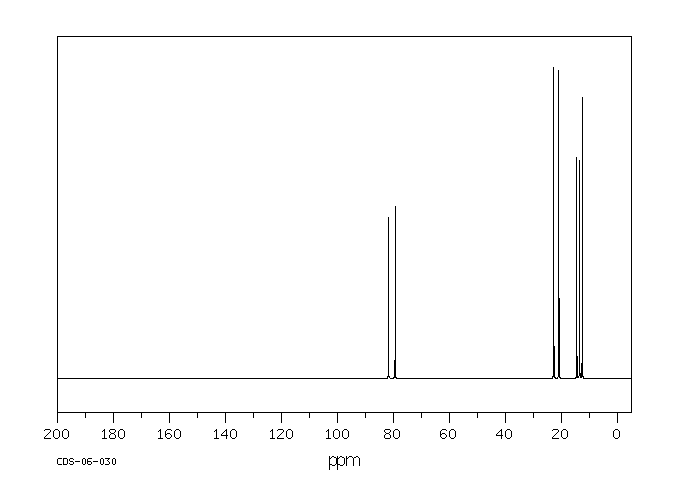
碳谱13CNMR
-
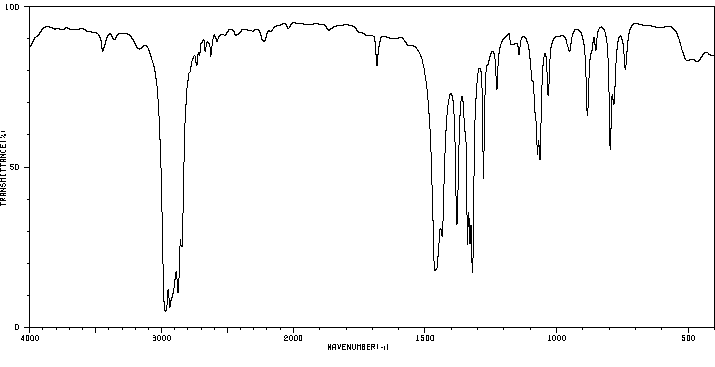
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-