3-溴3-甲基戊烷 | 25346-31-0
中文名称
3-溴3-甲基戊烷
中文别名
——
英文名称
3-bromo-3-methylpentane
英文别名
3-Brom-3-methyl-pentan
CAS
25346-31-0
化学式
C6H13Br
mdl
——
分子量
165.073
InChiKey
ZRPQYKLJSOLRPZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-92.2°C
-
沸点:147.26°C (estimate)
-
密度:1.1771
-
保留指数:916;929;944;876
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903399090
-
储存条件:存储条件:2-8℃,密封于干燥处。
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Selective Reduction of Alkyl Halides with Borohydride Exchange Resin-Nickel Acetate in Methanol摘要:DOI:10.1021/jo00095a054
-
作为产物:描述:参考文献:名称:Faworski; Ssakara, Zhurnal Russkago Fiziko-Khimicheskago Obshchestva, 1920, vol. 50, p. 58摘要:DOI:
文献信息
-
General method for iron-catalyzed multicomponent radical cascades–cross-couplings作者:Lei Liu、Maria Camila Aguilera、Wes Lee、Cassandra R. Youshaw、Michael L. Neidig、Osvaldo GutierrezDOI:10.1126/science.abj6005日期:2021.10.22addition to vinyl boronates) with Grignard reagents. Then, we extended the scope of these radical cascades by developing a general and broadly applicable Fe-catalyzed multicomponent annulation–cross-coupling protocol that engages a wide range of π-systems and permits the practical synthesis of cyclic fluorous compounds. Mechanistic studies are consistent with a bisarylated Fe(II) species being responsible
-
Fe-catalyzed three-component dicarbofunctionalization of unactivated alkenes with alkyl halides and Grignard reagents作者:Lei Liu、Wes Lee、Cassandra R. Youshaw、Mingbin Yuan、Michael B. Geherty、Peter Y. Zavalij、Osvaldo GutierrezDOI:10.1039/d0sc02127j日期:——reported. The reaction operates under fast turnover frequency and tolerates a diverse range of sp2-hybridized nucleophiles (electron-rich and electron-deficient (hetero)aryl and alkenyl Grignard reagents), alkyl halides (tertiary alkyl iodides/bromides and perfluorinated bromides), and unactivated olefins bearing diverse functional groups including tethered alkenes, ethers, protected alcohols, aldehydes
-
Nucleophilic Solvent Participation in the Solvolysis of Tertiary Bromoalkanes作者:Kwang-Ting Liu、Su-Jiun Hou、Meng-Lin TsaoDOI:10.1002/jccs.200900063日期:2009.4indicated limiting SN1 mechanism for the solvolysis. On the other hand, bromides 1B‐6B and 8B gave linear correlations (R = 0.987–0.996) with the dual‐parameter (YBr and NOTs) equation (2) only, which indicated the presence of significant nucleophilic solvent participation. Normal trends of reactivity due to the relief of B‐strain could be found in the poorly nucleophilic trifluoroethanol. Similar to the corresponding2-溴-2-甲基丙烷(1B),2-溴-2-甲基丁烷(2B),2-溴-2、3-二甲基丁烷(3B),2-溴-2、3、3-溶剂分解三甲基丁烷(4B),3-溴-3-甲基戊烷(5B),3-溴-2-,3-甲基戊烷(6B),3-溴- 2,2,3-三甲基戊烷(7B),3-溴-3-研究了15至21种溶剂中的乙基戊烷(8B),3-溴-3-乙基-2-甲基戊烷(9B)和2-溴-2、4、4-三甲基戊烷(11B),并通过使用检验了双参数Grunwald-Winstein方程(等式1和2)。基板7B,9B和11B表现出优异的线性关系(ř在日志≥0.997)ķ - ÿ溴图和表示限制性小号Ñ 1个机构,用于溶剂分解。另一方面,溴化物1B-6B和8B与双参数(Y Br和N OTs)具有线性相关性(R = 0.987-0.996))方程(2),表明存在大量亲核溶剂参与。在弱亲核性三氟乙醇中可以发现由于B菌株释放而引起的正常反应
-
Solvation effects in the heterolyses of 3‐X‐3‐methylpentanes (X = Cl, Br, I)作者:Filomena Martins、Ruben Elvas Leitão、Luís MoreiraDOI:10.1002/poc.816日期:2004.11A comparative study of the heterolysis reactions of 3-X-3-methylpentanes (X = Cl, Br, I) in a set of protic and aprotic solvents was performed at 25.00°C. Rate constant values were correlated with solvent descriptors using the TAKA multiparametric equation. Our results point towards a decrease in both hydrogen bond donor acidity (electrophilicity) and hydrogen bond acceptor basicity (nucleophilicity)
-
Three-Component Ru-Catalyzed Regioselective Alkylarylation of Vinylarenes via <i>Meta</i>-Selective C(sp<sup>2</sup>)–H Bond Functionalization作者:Hong-Chao Liu、Xiao-Ping Gong、Yu-Zhao Wang、Zhi-Jie Niu、Heng Yue、Xue-Yuan Liu、Yong-Min LiangDOI:10.1021/acs.orglett.2c00999日期:2022.4.29We report a novel Ru-catalyzed regioselective alkylarylation of vinylarenes with alkyl halides and arenes via meta-C(sp2)–H bond functionalization to construct 1,1-diarylalkanes that generally show bioactivity. In this transformation, a wide spectrum of primary, secondary, and tertiary alkyl halides and electronically varied arenes was well-tolerated. This reaction is characterized by its exquisite
表征谱图
-
氢谱1HNMR
-
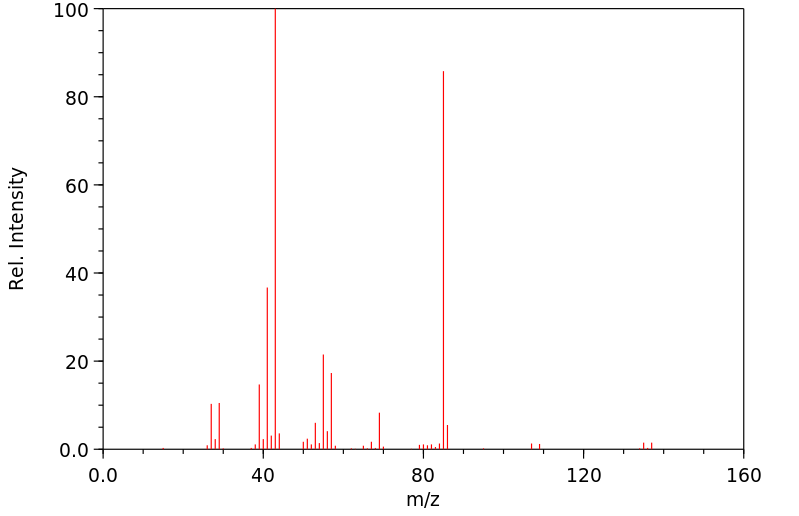
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4