4,5-二甲基-1,2-噻唑 | 27330-47-8
中文名称
4,5-二甲基-1,2-噻唑
中文别名
——
英文名称
4,5-Dimethyl-isothiazol
英文别名
4,5-dimethylisothiazole;4,5-dimethyl-1,2-thiazole
CAS
27330-47-8
化学式
C5H7NS
mdl
MFCD18451363
分子量
113.183
InChiKey
SPRVGONRQZESPA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:974;988;974
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:41.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
反应信息
-
作为反应物:描述:4,5-二甲基-1,2-噻唑 在 双氧水 作用下, 以 溶剂黄146 为溶剂, 反应 0.33h, 以80%的产率得到4,5-dimethyl-3-oxo-(2H)-isothiazole 1,1-dioxide参考文献:名称:Zur Oxidation von 1,2-Thiazolen:Ein einfacher Zugang zu 1,2-Thiazol-3(2 H)-on-1,1-dioxiden †摘要:1,2-噻唑的氧化; 1,2-Thiazol-3(2 H)-one 1,1-Dioxides的简便方法DOI:10.1002/hlca.19910740515
-
作为产物:描述:参考文献:名称:Muehlstaedt,M. et al., Journal fur praktische Chemie (Leipzig 1954), 1976, vol. 318, p. 507 - 514摘要:DOI:
-
作为试剂:描述:参考文献:名称:一种氯代苯氧羧酸胺盐的制备方法摘要:本发明提供了一种氯代苯氧羧酸胺盐的制备方法,包括以下步骤:S1)苯氧羧酸酯在催化剂A和催化剂B的作用下,和氯化剂进行2位和/或4位的选择性氯化反应,得到氯代苯氧羧酸酯;所述催化剂A为路易斯酸;所述催化剂B为C5~22的噻唑、异噻唑、噻吩或它们的卤代衍生物;S2)氯代苯氧羧酸酯和胺进行氨解反应,得到氯代苯氧羧酸胺盐。本发明通过对工艺路线的重新设计,对催化剂和氯化剂的精细筛选,降低了能耗,提高了氯化选择性,避免了有效成分的损失,所得氯代苯氧羧酸胺盐的收率可达99%以上。同时杜绝了高盐废水的产出,避免了氯代苯氧羧酸烘干及使用造成的粉尘危害,节约了能源,降低了设备投入。公开号:CN108947807A
文献信息
-
Thienoanellierte 6a?4-Thia-1,6-diazapentalene durch baseninduzierte Dimerisierung von 5-Methyl-isothiazoliumsalzen作者:B�rbel Schulze、Karen Rosenbaum、Jens Hilbig、Lutz WeberDOI:10.1002/prac.19923340105日期:——Isothiazolium salts 2 and 3 are easily available by reaction of (Z/E)-beta-thiocyanatovinyl aldehydes with primary aliphatic and aromatic amines in acetic acid or with aromatic amine hydrochlorides, respectively. Preparative advantages of this reaction are demonstrated and discussed. Reaction of 3 with secondary amines results in an unexpected formation of 6a-lambda-4-thia-1,6-diazapentalenes 5, a new typ of thiadiazapentalenes anellated with a heterocyclic ring system. The structure of 5 was evidenced by IR, UV, H-1-, C-13-n.m.r. spectral data and supported by elemental analysis. By means of N-15- and C-13-n.m.r. spectroscopy the synthesized thiadiazapentalenes were found to be stable towards protonation.
-
Muehlstaedt, Manfred; Schulze, Baerbel; Schubert, Ilona, Zeitschrift fur Chemie, 1981, vol. 21, # 9, p. 326 - 327作者:Muehlstaedt, Manfred、Schulze, Baerbel、Schubert, IlonaDOI:——日期:——
-
Schulze, Baerbel; Muehlstaedt, Manfred, Zeitschrift fur Chemie, 1988, vol. 28, # 10, p. 362作者:Schulze, Baerbel、Muehlstaedt, ManfredDOI:——日期:——
-
SCHULZE, B.;MUEHLSTAEDT, M.作者:SCHULZE, B.、MUEHLSTAEDT, M.DOI:——日期:——
-
SCHULZE, BARBEL;MUHLSTADT, MANFRED, Z. CHEM., 28,(1988) N 10, C. 362作者:SCHULZE, BARBEL、MUHLSTADT, MANFREDDOI:——日期:——
表征谱图
-
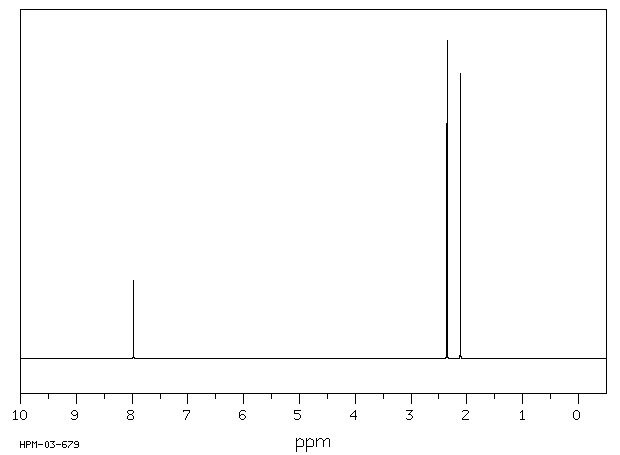
氢谱1HNMR
-
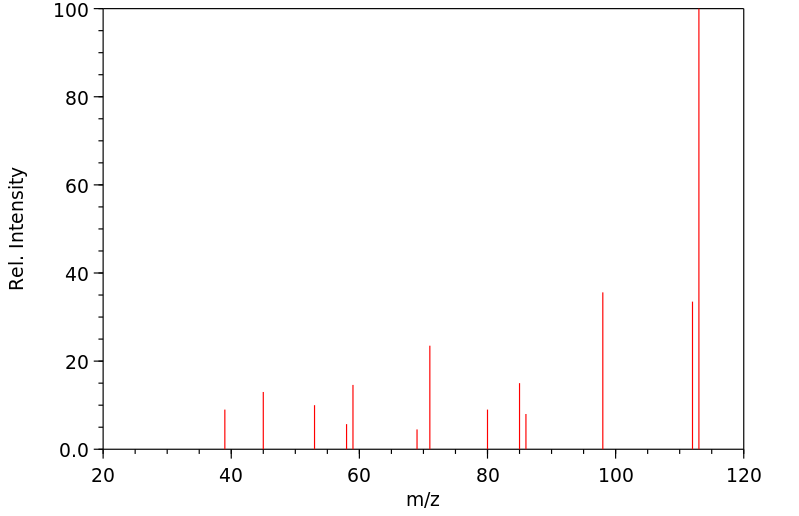
质谱MS
-
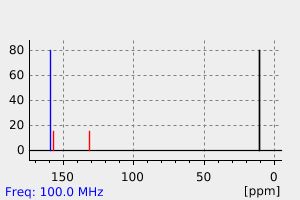
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)