碳酸丙烯乙酯 | 1469-70-1
中文名称
碳酸丙烯乙酯
中文别名
烯丙基乙基碳酸酯;碳酸烯丙基乙酯
英文名称
allyl ethyl carbonate
英文别名
ethyl allyl carbonate;Allylethyl carbonate;ethyl prop-2-enyl carbonate
CAS
1469-70-1
化学式
C6H10O3
mdl
——
分子量
130.144
InChiKey
BGSFCOHRQUBESL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:149°C
-
密度:0,99 g/cm3
-
稳定性/保质期:
如果按照规格使用和储存,不会发生分解,没有已知的危险反应。应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:3
-
安全说明:S16
-
危险类别码:R10
-
危险品运输编号:UN 3272
-
海关编码:2920909090
-
包装等级:III
-
危险类别:3
-
储存条件:请将贮藏器密封保存在阴凉干燥处,并确保工作环境有良好的通风或排气设施。
SDS
Allyl Ethyl Carbonate Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Allyl Ethyl Carbonate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Flammable liquid and vapour
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Allyl Ethyl Carbonate
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Allyl Ethyl Carbonate
Percent: >97.0%(GC)
CAS Number: 1469-70-1
Synonyms: Carbonic Acid Allyl Ethyl Ester
Chemical Formula: C6H10O3
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Allyl Ethyl Carbonate
Section 7. HANDLING AND STORAGE
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Almost colorless
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 149°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.99
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Allyl Ethyl Carbonate
Section 12. ECOLOGICAL INFORMATION
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
3: Flammable liquid.
Hazards Class:
UN-No: 3272
Esters, n.o.s.
Proper shipping name:
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Allyl Ethyl Carbonate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Flammable liquid and vapour
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Allyl Ethyl Carbonate
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Allyl Ethyl Carbonate
Percent: >97.0%(GC)
CAS Number: 1469-70-1
Synonyms: Carbonic Acid Allyl Ethyl Ester
Chemical Formula: C6H10O3
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Allyl Ethyl Carbonate
Section 7. HANDLING AND STORAGE
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Almost colorless
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 149°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.99
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Allyl Ethyl Carbonate
Section 12. ECOLOGICAL INFORMATION
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
3: Flammable liquid.
Hazards Class:
UN-No: 3272
Esters, n.o.s.
Proper shipping name:
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:Palladium(
II )-catalysed oxidation of carbon–carbon double bonds of allylic compounds with molecular oxygen; regioselective formation of aldehydes摘要:N-烯丙基酰胺1a–c和内酰胺1d–f在[PdCl2(MeCN)2]–CuCl催化剂存在下,于无水1,2-二氯乙烷含六甲基磷酰胺中与分子氧反应,可以区域选择性地得到相应的醛2,而在水的存在下,主要的产物是甲基酮3。DOI:10.1039/c39910001559 -
作为产物:参考文献:名称:设计,合成和构象活性的非天然桥接双环二肽作为高强度缺氧诱导因子-1抑制剂和抗肿瘤剂。摘要:通过基于棘霉素的双环肽结构进行结构修饰,我们成功合成了各种强大的抗肿瘤衍生物。通过与非天然键的交联限制了所得化合物中的环构象。所制备的衍生物被证明能强烈抑制缺氧诱导因子(HIF)-1的转录激活和HIF-1蛋白表达的缺氧诱导。特别是,烯烃桥连衍生物12表现出显着的细胞毒性(在MCF-7细胞系上,IC50 = 0.22 nM)和HIF-1抑制(IC50 = 0.09 nM),大大超过了棘霉素。构象分析和分子模型研究表明,通过构象限制,通过代谢稳定和刚性的桥键交联,生物活性得到增强。此外,我们提出了一种新的通过分子内π堆积稳定的球状构象,该构象可以促进双环二肽的生物效应。本研究中提出的进展为扩大药物发现中肽的化学空间提供了有用的指导。DOI:10.1021/acs.jmedchem.9b02039
-
作为试剂:描述:辛酸铑 、 4,5-双二苯基膦-9,9-二甲基氧杂蒽 在 碳酸丙烯乙酯 、 a-叠氮基-苯乙酸甲酯 、 caesium carbonate 、 N-甲基苯胺 作用下, 以 乙腈 为溶剂, 反应 9.0h, 生成 (5-(diphenylphosphanyl)-9,9-dimethyl-9H-xanthen-4-yl)diphenylphosphine oxide 、 、参考文献:名称:Dirhodium (II)/Xantphos 催化的中继卡宾插入和烯丙基烷基化过程:反应发展和机理洞察摘要:尽管重氮化合物、亲核试剂和亲电试剂的重铑催化多组分反应在有机合成方面取得了很大进展,但通过烯丙基金属中间体引入作为第三组分的烯丙基部分仍然是该领域的一项艰巨挑战。在此,公开了一种由新型 dirhodium (II)/Xantphos 催化实现的易于获得的胺、重氮化合物和烯丙基化合物的有吸引力的三组分反应,提供了各种结构复杂且功能多样的 α-季铵 α-氨基酸衍生物。具有高原子和步骤经济的产量。机理研究表明,该转化是通过中继二铑(II)催化的卡宾插入和烯丙基烷基化过程实现的,DOI:10.1021/jacs.1c05701
文献信息
-
[EN] NEW CATIONIC DYES, KITS AND COMPOSITIONS THEREOF, AND PROCESS FOR DYEING KERATIN FIBERS<br/>[FR] NOUVEAUX COLORANTS CATIONIQUES, KITS ET COMPOSITIONS LES CONTENANT, ET PROCÉDÉ DE TEINTURE DE FIBRES KÉRATINIQUES申请人:ALFA PARF GROUP S P A公开号:WO2014202150A1公开(公告)日:2014-12-24The present invention relates to new cationic dyes of general formula (I) and (II): The invention also relates to kits and compositions for dyeing keratin fibers, which contain at least one of these dyes as well as to a process for dyeing keratin fibers using at least one of these dyes.
-
[EN] PRMT5 INHIBITORS CONTAINING A DIHYDRO- OR TETRAHYDROISOQUINOLINE AND USES THEREOF<br/>[FR] INHIBITEURS DE LA PRMT5 CONTENANT UNE DIHYDRO- OU TÉTRAHYDRO-ISOQUINOLÉINE ET LEURS UTILISATIONS申请人:EPIZYME INC公开号:WO2014100730A1公开(公告)日:2014-06-26Described herein are compounds of Formula (A), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof. Compounds of the present invention are useful for inhibiting PRMT5 activity. Methods of using the compounds for treating PRMT5- mediated disorders are also described.
-
PRMT5 INHIBITORS AND USES THEREOF申请人:Duncan Kenneth W.公开号:US20190083482A1公开(公告)日:2019-03-21Described herein are compounds of Formula (I), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof. Compounds of the present invention are useful for inhibiting PRMT5 activity. Methods of using the compounds for treating PRMT5-mediated disorders are also described.
-
D-AMINO ACID OXIDASE INHIBITORS AND THERAPEUTIC USES THEREOF申请人:Tsai Guochuan Emil公开号:US20190112289A1公开(公告)日:2019-04-18The present invention relates to compounds of Formula (I): or a pharmaceutically acceptable salt thereof, wherein: each of A, B, C, D, and E, independently, is C, N, N—H, O, S, or absent is a single bond or a double bond; each of X, Y, and Z, independently, is aryl, heteroaryl, aralkyl, H, or absent; each of L 1 and L 2 , independently, is a moiety selected from O, CH 2 , C═O, C 2-10 alkyl, C 2-10 alkenyl, C 2-10 alkynyl, —((CH 2 ) n —W)—, wherein n=0, 1, 2, 3, 4, or 5, and W is O or S, or absent; and when L 2 is absent, Z is aryl or heteroaryl fused with B C. Also provided in the present invention is a method for inhibiting, treating and/or reducing the risk of a neuropsychiatric disorder, comprising administering a subject in need a composition comprising a compound of Formula (I).本发明涉及以下式(I)的化合物: 或其药学上可接受的盐,其中:A、B、C、D 和 E 中的每一个独立地是 C、N、N—H、O、S 或不存在 是单键或双键;X、Y 和 Z 中的每一个独立地是芳基、杂环芳基、芳基烷基、H 或不存在;L 1 和 L 2 中的每一个独立地是从 O、CH 2 、C═O、C 2-10 烷基、C 2-10 烯基、C 2-10 炔基、—((CH 2 ) n —W)— 中选择的基团,其中 n=0、1、2、3、4 或 5,W 是 O 或 S,或不存在;当 L 2 不存在时,Z 是与 B 相融合的芳基或杂环芳基。本发明还提供了一种用于抑制、治疗和/或减少神经精神障碍风险的方法,包括向需要的受试者施用包含式(I)化合物的组合物。
-
[EN] PEPTIDE-BASED MULTIPLE-DRUG DELIVERY VEHICLE<br/>[FR] VÉHICULE D'ADMINISTRATION DE MÉDICAMENTS MULTIPLES À BASE DE PEPTIDES申请人:ARIEL-UNIVERSITY RES AND DEV COMPANY LTD公开号:WO2017068577A1公开(公告)日:2017-04-27A molecular structure comprising a targeting moiety, a multi-functional peptide platform and a plurality of controllably released bioactive agents attached thereto is provided herein.本文提供了一种包括靶向基团、多功能肽平台和附着在其上的多种可控释放的生物活性剂的分子结构。
表征谱图
-
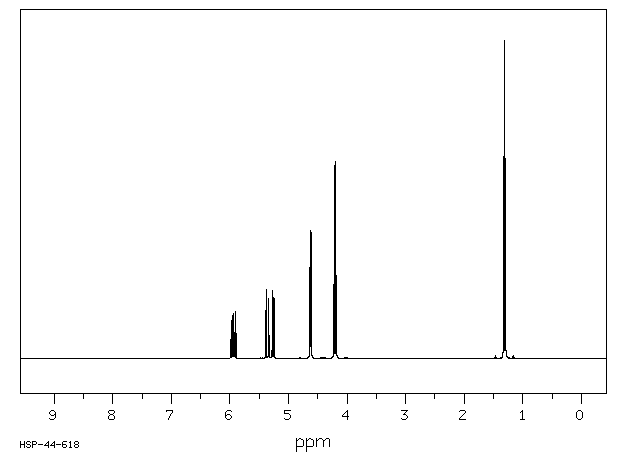
氢谱1HNMR
-
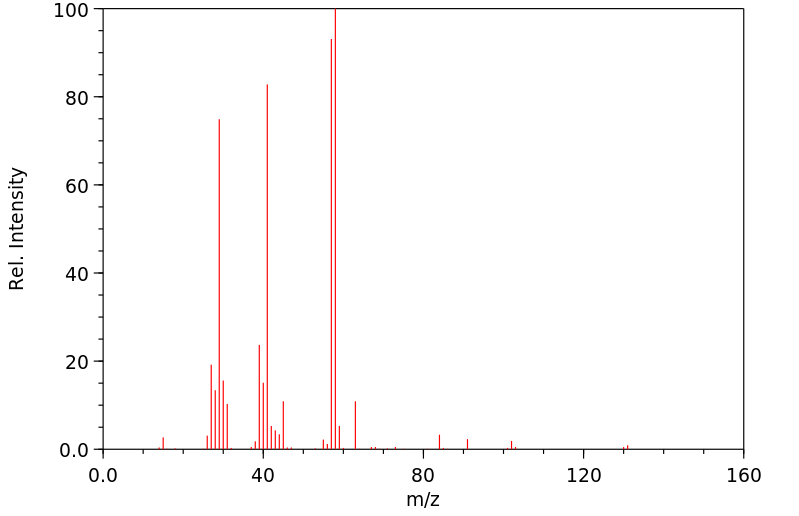
质谱MS
-
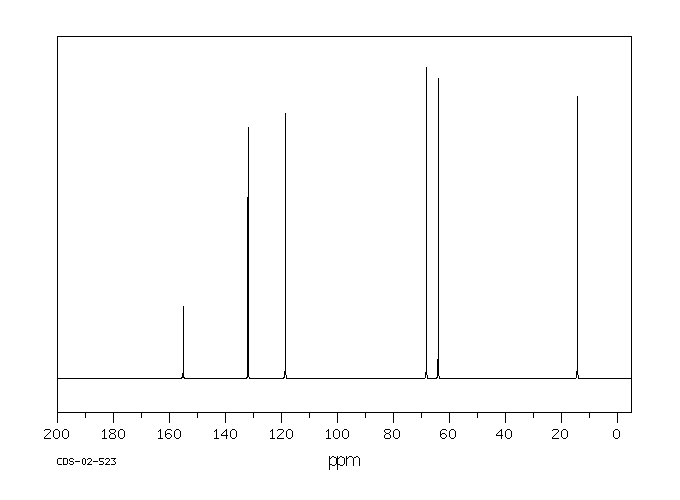
碳谱13CNMR
-
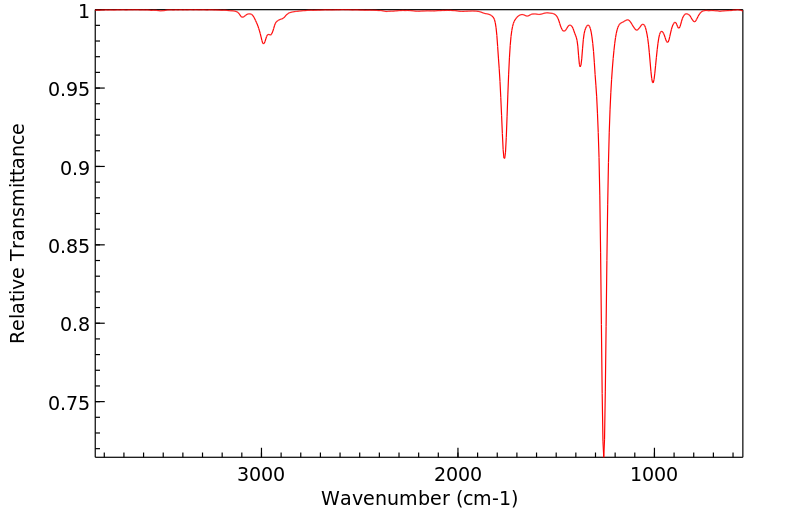
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-4-[(甲基氨基甲酰)氨基]环己烷羧酸
顺式-3-己烯醇碳酸甲酯
镏碳酸盐二水
镍,[碳酸(2-)-κO]-
镁(1-甲基-3-氧代-丁-1-烯基)碳酸氢酯
锌氮烷碳酸盐
锆碳酸盐氧化物
锂(1-羧基环丙基)锂
铵铜碳酸盐
铯碳酸氢钠
铝镁加
铝镁加
铝碳酸镁
铝碳酸镁
钠脲氯酸盐
钠甲基碳酸酯
钙钠碳酸氢盐氟化物
钙四镁钠碳酸氢盐三碳酸盐四氢氧化物
钐(+3)阳离子碳酸酯
重质碳酸镁
重碳酸钠-13C
酸氧(-2)阴离子铅杂亚酸碳
酮羧酸
邻苯二甲酸氢壬酯
过氧碳酸钠
过氧碳酸二钠盐
过氧碳酸,O,O'-1,6-亚己基-OO,OO'-二叔丁基酯
过氧化脲素
过氧化二碳酸双十四酯
过氧化二碳酸双十六酯
过氧化二碳酸二硬脂酰酯
过氧化二碳酸二环己酯
过氧化二碳酸二正丁酯
过氧化二碳酸二异丙酯
过氧化二碳酸二仲丁酯
过氧化二碳酸二乙酯
过氧化二碳酸二-3-甲氧基丁酯
过氧化二碳酸二(2-乙基己)酯
过氧化(2-乙基己基)碳酸叔戊酯
过氧二碳酸二十三烷酯
过氧二碳酸二丙基酯
达比加群酯杂质41
达比加群酯杂质22
达比加群杂质36
达比加群杂质19
辛酰脲
辛基辛氧基甲基碳酸酯
辛基脲
轻质碳酸镁
起始原料2杂质B