4-羟基-3-硝基苄醇 | 41833-13-0
物质功能分类
中文名称
4-羟基-3-硝基苄醇
中文别名
3-硝基-4羟基苄醇
英文名称
4-hydroxymethyl-2-nitrophenol
英文别名
4-hydroxy-3-nitrobenzyl alcohol;4-(hydroxymethyl)-2-nitrophenol
CAS
41833-13-0
化学式
C7H7NO4
mdl
——
分子量
169.137
InChiKey
IMLGJYRKLCMJPI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:94-98 °C
-
沸点:298.4°C (rough estimate)
-
密度:1.4523 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:86.3
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn,Xi
-
危险类别码:R36/37/38
-
海关编码:2908999090
-
安全说明:S26,S36/37/39
-
危险性防范说明:P305+P351+P338
-
危险性描述:H319
-
储存条件:存放于阴凉干燥处即可。
SDS
| Name: | 4-Hydroxy-3-nitrobenzyl alcohol Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 41833-13-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 41833-13-0 | 4-Hydroxy-3-nitrobenzyl alcohol | 100 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 41833-13-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light brown - bright yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H7NO4
Molecular Weight: 169.0533
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 41833-13-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-Hydroxy-3-nitrobenzyl alcohol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 41833-13-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 41833-13-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 41833-13-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-羟基-3-硝基苯甲酸 3-nitro-4-hydroxybenzoic acid 616-82-0 C7H5NO5 183.12 4-羟基-3-硝基苯甲醛 4-hydroxy-3-nitro-benzaldehyde 3011-34-5 C7H5NO4 167.121 4-氯甲基-2-硝基苯酚 3-Nitro-4-hydroxybenzyl chloride 6694-75-3 C7H6ClNO3 187.583 2-氨基-4-(羟基甲基)苯酚 2-amino-4-hydroxymethylphenol 52820-13-0 C7H9NO2 139.154 硝苯酚 2-hydroxynitrobenzene 88-75-5 C6H5NO3 139.111 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(methoxymethyl)-2-nitrophenol —— C8H9NO4 183.164 4-甲氧基-3-硝基苯甲基醇 (4-methoxy-3-nitrophenyl)methanol 41870-24-0 C8H9NO4 183.164 —— 4-hydroxy-3-nitrobenzyl acetate 32645-00-4 C9H9NO5 211.174 4-羟基-3-硝基苯甲酸 3-nitro-4-hydroxybenzoic acid 616-82-0 C7H5NO5 183.12 (4-乙氧基-3-硝基苯基)甲醇 4-ethoxy-3-nitro-benzyl alcohol 697304-05-5 C9H11NO4 197.191 4-羟基-3-硝基苯甲醛 4-hydroxy-3-nitro-benzaldehyde 3011-34-5 C7H5NO4 167.121 4-氯甲基-2-硝基苯酚 3-Nitro-4-hydroxybenzyl chloride 6694-75-3 C7H6ClNO3 187.583 —— 4-hydroxy-3-nitrobenzyl bromide 177179-94-1 C7H6BrNO3 232.034 —— [4-({[4-(methyloxy)phenyl]methyl}oxy)-3-nitrophenyl]methanol 942215-76-1 C15H15NO5 289.288 4-羟甲基-2-硝基苯乙酸甲酯醚 methyl 2-[4-(hydroxymethyl)-2-nitrophenoxy]acetate 308815-81-8 C10H11NO6 241.2 3-硝基-4-甲氧基苄溴 3-nitro-4-methoxybenzyl bromide 61010-34-2 C8H8BrNO3 246.06 2-氨基-4-(羟基甲基)苯酚 2-amino-4-hydroxymethylphenol 52820-13-0 C7H9NO2 139.154 —— 2-amino-(4-ethoxymethyl)phenol 534571-75-0 C9H13NO2 167.208 - 1
- 2
反应信息
-
作为反应物:描述:4-羟基-3-硝基苄醇 在 manganese (II) nitrate tetrahydrate 、 copper(II) nitrate trihydrate 、 溶剂黄146 作用下, 以 三氟甲苯 为溶剂, 反应 2.0h, 以91%的产率得到4-羟基-3-硝基苯甲醛参考文献:名称:TEMPO包覆的Fe3O4超顺磁性纳米粒子的简单制备及其在酒精选择性氧化中的应用摘要:通过使用强金属氧化物螯合膦酸酯和叠氮化物/炔烃“咔嗒”化学反应,将有机氧化剂TEMPO(2,2,4,4-四甲基哌啶-1-氧基)固定在氧化铁(Fe 3 O 4)超顺磁性纳米粒子上。这种简单的制备方法可产生具有良好TEMPO负载的可回收TEMPO涂层纳米颗粒。它们具有出色的磁响应,并能在需氧酸性Mn II / Cu II下有效地催化多种伯醇和仲醇氧化成醛,酮和内酯。氧化Minisci条件或碱性NaOCl Anelli条件。纳米颗粒在Minisci条件下可以循环使用20次以上,在Anelli条件下可以循环使用八次,具有良好的基材转化率和优异的产品选择性。通过膦酸酯键固定化催化剂可使颗粒在最少的催化剂浸出的情况下承受酸性氧化环境。在固定膦酸酯之前而不是之后,将TEMPO单击膦酸酯,以确保单击的催化剂是颗粒表面上唯一的物质。这有助于定量催化剂负载。膦酸酯连接基的稳定性和这种催化剂固定方法的简便性使其DOI:10.1002/chem.200903527
-
作为产物:描述:参考文献:名称:碘(III)通过非布朗斯台德酸性NO 2 +生成的苯酚亲电硝化摘要:使用碘基苯作为基于碘(III)和硝酸铝作为硝基基团来源的有机催化剂,开发了用于苯酚亲电硝化的第一个催化程序。该原子经济方案发生在温和的,非布朗斯台德酸性和开放式烧瓶反应条件下,具有宽泛的官能团耐受性,包括多个杂环。(SMD:MeCN)Mo8-HX /(LANLo8 + f,6-311 + G *)水平的密度泛函理论(DFT)计算表明反应通过阳离子途径进行,该途径有效地产生了NO 2 +离子,从而是中性条件下的硝化物种。DOI:10.1021/acs.orglett.8b04141
文献信息
-
Heterocyclic ketones申请人:ICI Americas Inc.公开号:US05164371A1公开(公告)日:1992-11-17The invention provides a series of novel heterocyclic ketones of formula I ##STR1## and pharmaceutically acceptable base-addition salts thereof, in which the values of R.sup.4, L, A, X and Q have the meanings defined in the following specification. The compounds of formula I are inhibitors of human leukocytic elastase. The invention also provides pharmaceutical compositions containing a compound of formula I, or a pharmaceutically acceptable base-addition salt thereof, and processes and intermediates for the manufacture of compounds of formula I.
-
Prodrugs of a JAK Inhibitor Compound for Treatment of Gastrointestinal Inflammatory Disease申请人:THERAVANCE BIOPHARMA R&D IP, LLC公开号:US20170145044A1公开(公告)日:2017-05-25The invention provides compounds which are prodrugs of a JAK inhibitor agent for the targeted delivery of the JAK inhibitor to the gastrointestinal tract of a mammal. The invention also provides pharmaceutical compositions comprising the compounds, methods of using the compounds to treat gastrointestinal inflammatory diseases, and processes and intermediates useful for preparing the compounds.本发明提供了一种前药化合物,它是针对哺乳动物胃肠道靶向输送JAK抑制剂的前药。本发明还提供了包含该化合物的药物组合物,使用该化合物治疗胃肠道炎症性疾病的方法,以及用于制备该化合物的过程和中间体。
-
[EN] COMPOSITIONS AND METHODS RELATED TO ANTI-CD19 ANTIBODY DRUG CONJUGATES<br/>[FR] COMPOSITIONS ET MÉTHODES ASSOCIÉES À DES CONJUGUÉS ANTICORPS ANTI-CD19-MÉDICAMENTS申请人:LEGOCHEM BIOSCIENCES INC公开号:WO2017051249A1公开(公告)日:2017-03-30In some aspects, the invention relates to an antibody-drug conjugate, comprising an anti-CD 19 antibody; a linker; and an active agent. The antibody-drug conjugate may comprise a self-immolative group. The linker may comprise an O-substituted oxime, e.g., wherein the oxygen atom of the oxime is substituted with a group that covalently links the oxime to the active agent; and the carbon atom of the oxime is substituted with a group that covalently links the oxime to the antibody.
-
[EN] COMPOSITIONS AND METHODS RELATED TO ANTI-EGFR ANTIBODY DRUG CONJUGATES<br/>[FR] COMPOSITIONS ET MÉTHODES ASSOCIÉES À DES CONJUGUÉS ANTICORPS ANTI-EGFR-MÉDICAMENTS申请人:LEGOCHEM BIOSCIENCES INC公开号:WO2017051254A1公开(公告)日:2017-03-30In some aspects, the invention relates to an antibody-drug conjugate, comprising an anti-epidermal growth factor receptor ("EGFR") antibody; a linker; and an active agent. The antibody-drug conjugate may comprise a self-immolative group. The linker may comprise an O-substituted oxime, e.g., wherein the oxygen atom of the oxime is substituted with a group that covalently links the oxime to the drug; and the carbon atom of the oxime is substituted with a group that covalently links the oxime to the antibody.
-
IRON OXIDE SUPPORTED RHODIUM CATALYST FOR NITROARENE REDUCTION申请人:King Fahd University of Petroleum and Minerals公开号:US20200298212A1公开(公告)日:2020-09-24A supported catalyst having rhodium particles with an average diameter of less than 1 nm disposed on a support material containing magnetic iron oxide (e.g. Fe 3 O 4 ). A method of producing the supported catalyst and a process of reducing nitroarenes to corresponding aromatic amines employing the supported catalyst with a high product yield are also described. The supported catalyst may be recovered with ease using an external magnet and reused.
表征谱图
-
氢谱1HNMR
-
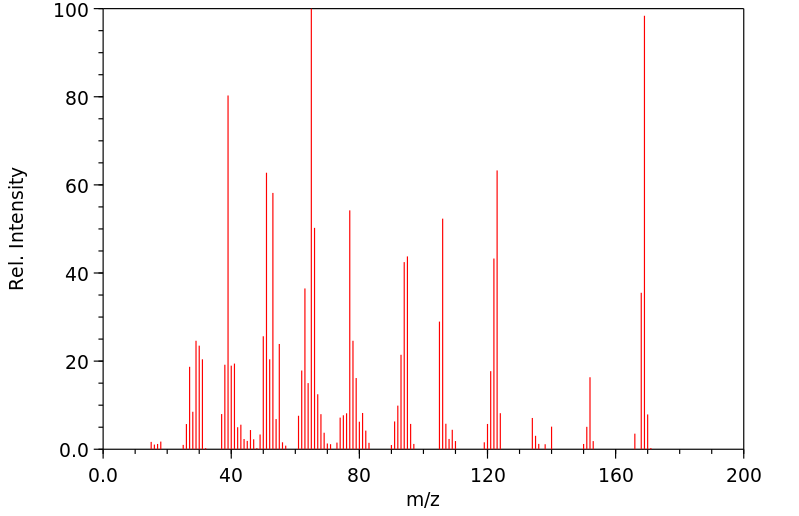
质谱MS
-
碳谱13CNMR
-
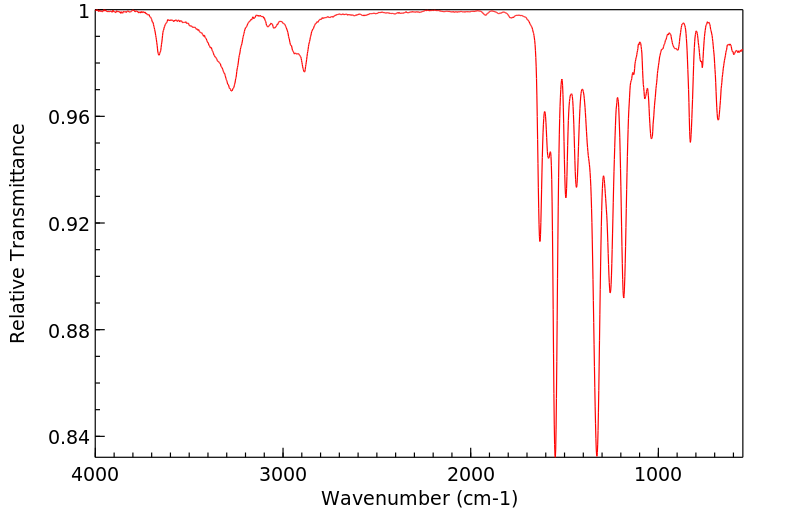
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚