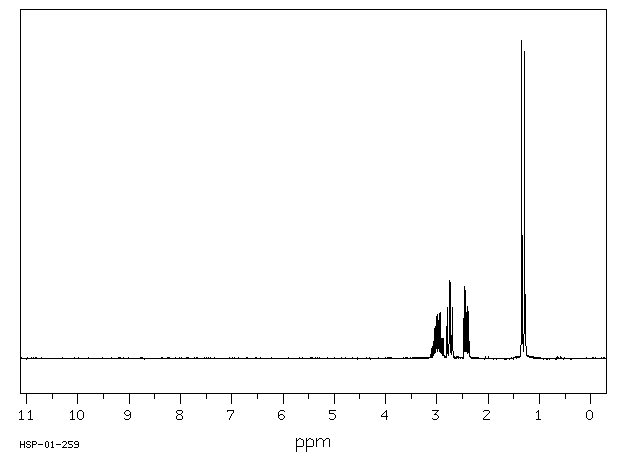
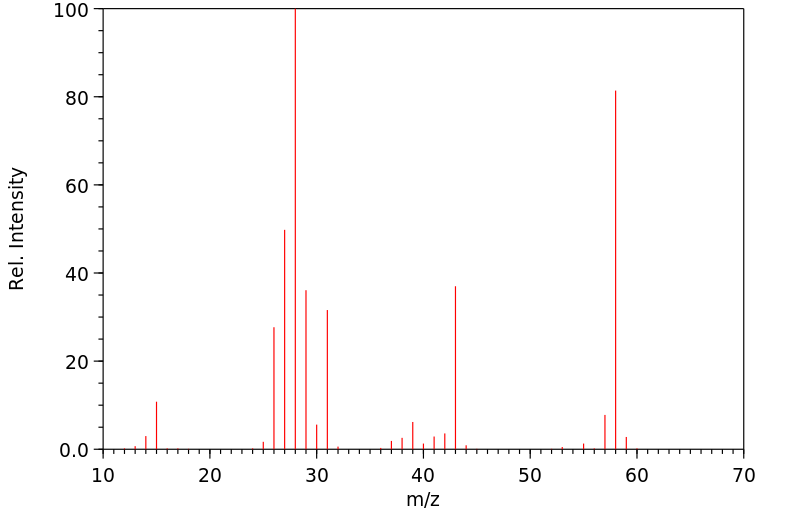
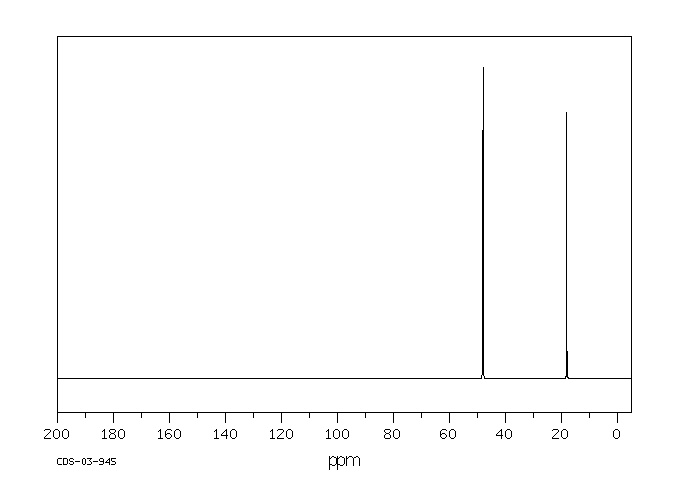
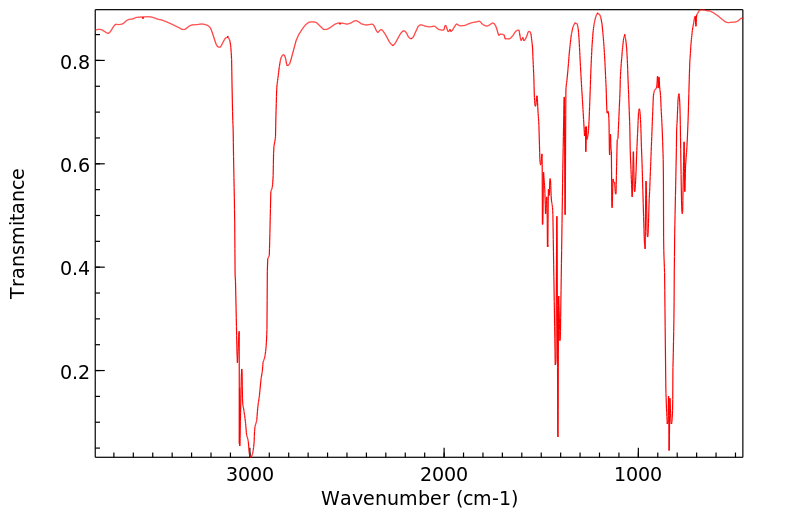
代谢
环氧
丙烷在中性pH下与DNA反应,生成两个主要产物,即N-7-(2-羟基丙基)
鸟嘌呤和N-3-(2-羟基丙基)
腺嘌呤。
来源:Hazardous Substances Data Bank (HSDB)
代谢
在一家生产烷基淀粉的工厂中,对暴露于环氧
丙烷的工人的淋巴细胞进行了染色体畸变和微核的测量,同时也对与医疗器械消毒相关的
环氧乙烷暴露工人进行了测量。血红蛋白中的加合物
水平被用来确定两种化合物的体内剂量。环氧
丙烷暴露工人的羟丙基缬
氨酸
水平与估计的暴露剂量相关。未暴露组中这种加合物的
水平接近于检测方法的检测限。环氧
丙烷暴露组中记录的羟乙基缬
氨酸
水平与早期关于职业未暴露受试者血红蛋白烷基化的数据一致。加合物测量结果显示,两组
环氧乙烷暴露工人(即低暴露的装配工和高暴露的消毒工)的羟乙基缬
氨酸
水平升高,符合预期的是,这两个亚组的加合物
水平不同。细胞遗传学研究的结果显示,环氧
丙烷的致裂变潜力低于
环氧乙烷,因为尽管最后一组的加合物
水平较低,但环氧
丙烷暴露个体的微核和染色体断裂频率低于装配工。
来源:Hazardous Substances Data Bank (HSDB)
代谢
聚
丙烯及其活性代谢物环氧
丙烷在大鼠体内的药代动力学进行了评估,以检查两种化合物在致癌性方面的所谓差异。斯普拉格-道利大鼠分组在封闭式暴露系统中暴露于
丙烯或环氧
丙烷。药代动力学参数由双室模型确定,该模型用于计算吸入
丙烯后产生的环氧
丙烷的身体负担。整个身体与空气比例的热力学平衡常数取决于物质的物理性质,确定为
丙烯为1.6,环氧
丙烷为45。根据米氏常数,发现
丙烯具有饱和动力学;相比之下,在暴露室的空气中初始浓度高达3000 ppm时,环氧
丙烷没有观察到饱和动力学。通过药代动力学参数,计算了在连续暴露于环氧
丙烷或
丙烯的不同条件下环氧
丙烷的身体负担。如果大鼠即使在高浓度下暴露于
丙烯,环氧
丙烷的最大身体负担也不会超过每毫升组织71纳升环氧
丙烷气体的浓度。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
癌症分类:B2组可能的人类致癌物
来源:Hazardous Substances Data Bank (HSDB)
毒理性
分类:B2;可能的人类致癌物。分类依据:基于不充分的人类数据和两种动物接触部位在皮下注射、吸入和灌胃暴露时良性及恶性肿瘤发生率增加的情况。还有证据显示在多种测试系统中具有诱变性。环氧
丙烷的结构与其他在动物中显示致癌活性的
化学品相似。人类致癌性数据:不充分。动物致癌性数据:充分。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:对于环氧
丙烷对人类致癌性的证据不足。对于环氧
丙烷对实验动物致癌性的证据是充分的。总体评估:环氧
丙烷可能对人类具有致癌性(2B组)。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A3:已确认的动物致癌物,对人类的相关性未知。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
呼吸系统是吸收的主要途径,尽管皮肤也代表了一个生理上重要的物质进入身体的途径...。
来源:Hazardous Substances Data Bank (HSDB)