5-(2-氨基乙基氨基)-1-萘磺酸 | 50402-56-7
中文名称
5-(2-氨基乙基氨基)-1-萘磺酸
中文别名
5-(2-氨基乙氨基)-1-萘磺酸;5-(2-氨基乙胺)-1-萘磺酸;N(氨乙基)-5-萘胺-1-磺酸
英文名称
5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid
英文别名
EDANS;5-[(2-aminoethyl)amino]-1-naphthalenesulfonic acid;5-((2-aminoethyl)-amino)-naphtalene-1-sulfonic acid;5-(2-Azaniumylethylamino)naphthalene-1-sulfonate
CAS
50402-56-7
化学式
C12H14N2O3S
mdl
MFCD00066932
分子量
266.321
InChiKey
SJQRQOKXQKVJGJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>350°C
-
密度:1.432±0.06 g/cm3(Predicted)
-
溶解度:可溶于水基(轻微),DMSO(轻微,加热)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):-1.3
-
重原子数:18
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:101
-
氢给体数:3
-
氢受体数:5
安全信息
-
危险品标志:Xn
-
安全说明:S26,S27,S36/37/39,S45
-
危险类别码:R22,R36/37/38,R43
-
WGK Germany:3
-
海关编码:2921590090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H317,H319,H335
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 5-(2-Aminoethylamino)-1-naphthalenesulfonic acid
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 50402-56-7
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 4), H302
Skin irritation (Category 2), H315
Eye irritation (Category 2), H319
Skin sensitisation (Category 1), H317
Specific target organ toxicity - single exposure (Category 3), H335
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xn Harmful R22, R36/37/38, R43
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Warning
Hazard statement(s)
H302 Harmful if swallowed.
H315 Causes skin irritation.
H317 May cause an allergic skin reaction.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust.
P280 Wear protective gloves.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Chemical characterization : Natural product
Synonyms : N-(Aminoethyl)-5-naphthylamine-1-sulfonic acid
1,5-EDANS
Formula : C12H14N2O3S
Molecular Weight : 266,32 g/mol
CAS-No. : 50402-56-7
EC-No. : 256-575-5
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
5-(2-Aminoethylamino)naphthalene-1-sulphonic acid
CAS-No. 50402-56-7 Acute Tox. 4; Skin Irrit. 2; Eye <= 100 %
EC-No. 256-575-5 Irrit. 2; Skin Sens. 1; STOT SE
3; H302, H315, H317, H319,
H335
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
5-(2-Aminoethylamino)naphthalene-1-sulphonic acid
CAS-No. 50402-56-7 Xn, R22 - R36/37/38 - R43 <= 100 %
EC-No. 256-575-5
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx), Sulphur oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Full contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
Splash contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374
If used in solution, or mixed with other substances, and under conditions which differ from EN 374,
contact the supplier of the CE approved gloves. This recommendation is advisory only and must
be evaluated by an industrial hygienist and safety officer familiar with the specific situation of
anticipated use by our customers. It should not be construed as offering an approval for any
specific use scenario.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 300 °C
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-[2-(Hex-5-ynoylamino)ethylamino]naphthalene-1-sulfonic acid 1167424-99-8 C18H20N2O4S 360.434 —— 5-[2-[(2-Methylpropan-2-yl)oxycarbonylamino]ethylamino]naphthalene-1-sulfonic acid 884520-64-3 C17H22N2O5S 366.438 —— 5-[N-(2-(((trimethylsilyl)methyl)amino)ethyl)-N-((trimethylsilyl)methyl)amino]naphthalene-1-sulfonic acid 886587-01-5 C20H34N2O3SSi2 438.739 —— 5-[N-(2-(N-methyl-N-((trimethylsilyl)methyl)amino)ethyl)-N-((trimethylsilyl)methyl)amino]naphthalene-1-sulfonic acid 886587-02-6 C21H36N2O3SSi2 452.765 —— 5-((2-(4-azidobenzamido)ethyl)amino)naphthalene-1-sulfonic acid 1375413-65-2 C19H17N5O4S 411.441 —— 4-[3-Methoxy-4-[[2-[(5-sulfonaphthalen-1-yl)amino]ethylamino]methyl]phenoxy]butanoic acid 816430-11-2 C24H28N2O7S 488.562 —— Boc-Glu(EDANS)-OBzl 143774-97-4 C29H35N3O8S 585.678
反应信息
-
作为反应物:描述:5-(2-氨基乙基氨基)-1-萘磺酸 在 palladium on activated charcoal 盐酸 、 氢气 、 (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate 、 N,N-二异丙基乙胺 作用下, 以 1,4-二氧六环 、 甲醇 、 N,N-二甲基甲酰胺 为溶剂, 25.0 ℃ 、275.79 kPa 条件下, 反应 15.0h, 生成 Boc-GABA-Glu(EDANS)-OH参考文献:名称:使用固相肽合成制备内部淬灭的荧光蛋白酶底物的一般方法。摘要:提出了通过固相合成方法获得荧光供体/受体肽底物的一般方案。此方法的关键特征是设计谷氨酸衍生物,该谷氨酸衍生物已在羧基侧链上被5-[((2'-氨基乙基)-氨基]萘磺酸磺酸(EDANS)修饰,以生成可以可以在肽底物的C-末端附近结合。然后可以将包含4-[[[4-(二甲基氨基)苯基]偶氮]苯甲酸(DABCYL)的相应荧光受体基团在N末端连接到树脂结合的肽上,同时所有侧链基团仍被完全保护。 。合成了肾素和HIV蛋白酶的底物。DOI:10.1021/jm00099a001
-
作为产物:描述:参考文献:名称:荧光双功能反式环辛烯作为研究点击释放动力学的有效工具。摘要:四嗪和烯丙基取代的反式环辛烯(TCO)之间的逆电子需求狄尔斯-阿尔德哒嗪消除反应是生物正交键断裂反应的关键参与者。迄今为止,确定烷基胺底物的消除率很困难。在这里,我们报告了一种由 TCO 连接的 EDANS 荧光团和 DABCYL 猝灭剂组成的荧光工具,用于准确测定任何四嗪在生理相关浓度下的点击和释放速率常数。DOI:10.1002/chem.201905446
文献信息
-
[EN] TARGETED DRUG DELIVERY THROUGH AFFINITY BASED LINKERS<br/>[FR] ADMINISTRATION CIBLÉE D'UN MÉDICAMENT FAISANT APPEL À DES COUPLEURS FONDÉS SUR L'AFFINITÉ申请人:INVICTUS ONCOLOGY PVT LTD公开号:WO2015148126A1公开(公告)日:2015-10-01The current invention discloses targeted drug delivery conjugates comprising a targeting moiety linked to a drug via a molecule having an affinity for the targeting moiety. Typically, the conjugate comprises a targeting ligand and a molecule of interest, e.g., a therapeutic agent. The targeting ligand and the molecule of interest are linked to each other via an affinity ligand. The affinity ligand is further covalently or non-covalently linked to a drug or therapeutic agent. The drug can be modified to make it more soluble and so that it cleaves from the linking molecule at the target site.
-
[EN] COMBINATION PHARMACEUTICAL AGENTS AS RSV INHIBITORS<br/>[FR] AGENTS PHARMACEUTIQUES EN COMBINAISON EN TANT QU'INHIBITEURS DE RSV申请人:ENANTA PHARM INC公开号:WO2019067864A1公开(公告)日:2019-04-04The present invention relates to pharmaceutical agents administered to a subject either in combination or in series for the treatment of a Respiratory Syncytial Virus (RSV) infection, wherein treatment comprises administering a compound effective to inhibit the function of the RSV and an additional compound or combinations of compounds having anti-RSV activity.本发明涉及用于治疗呼吸道合胞病毒(RSV)感染的药物剂,该药物剂可以单独或连续给予受试者,治疗包括给予一种有效抑制RSV功能的化合物以及具有抗RSV活性的另一种化合物或化合物组合。
-
[EN] CHEMOSELECTIVE THIOL-CONJUGATION WITH ALKENE OR ALKYNE-PHOSPHONAMIDATES<br/>[FR] CONJUGAISON CHIMIOSÉLECTIVE D'UN THIOL AVEC DES ALCÈNE- OU ALCYNE-PHOSPHONAMIDATES申请人:FORSCHUNGSVERBUND BERLIN EV公开号:WO2018041985A1公开(公告)日:2018-03-08Disclosed are novel conjugates and processes for the preparation thereof. A process for the preparation of alkene- or alkyne-phosphonamidates comprises the steps of (I) reacting a compound of formula (III), with an azide of formula (IV), to prepare a compound of formula (V), reacting a compound of formula (V) with a thiol-containing molecule of formula (VI), resulting in a compound of formula (VII).
-
LIGHT-ENABLED DRUG DELIVERY申请人:Virginia Commonwealth University公开号:US20140236071A1公开(公告)日:2014-08-21Conjugates are provided which comprise a membrane permeable drug linked to a moiety that is not membrane permeable. Attachment of the moiety that is not membrane permeable prevents the drug from crossing cell membranes and entering cells. However, exposure to light either i) breaks the linkage, releasing the drug and allowing it to enter cells; or ii) converts the non-membrane permeable moiety to a membrane permeable form, allowing the entire conjugate to enter the cell, where the drug is released from the conjugate by cleavage. The membrane permeable drugs are thus delivered to cells at locations of interest, e.g. cancer cells in a tumor, in a temporally and spatially controlled manner.提供了一种缀合物,该缀合物包括与一个不可透过细胞膜的基团相连的可透过细胞膜的药物。不可透过细胞膜的基团的附着阻止了药物穿过细胞膜并进入细胞。然而,光照要么i)断裂这种连接,释放药物并允许其进入细胞;要么ii)将不可透过细胞膜的基团转化为可透过细胞膜的形式,允许整个缀合物进入细胞,在细胞中药物通过裂解从缀合物中释放。因此,可透过细胞膜的药物以时间和空间可控的方式被递送到感兴趣的位置,例如肿瘤中的癌细胞。
-
MACROCYCLIC, PYRIDAZINONE-CONTAINING HEPATITIS C SERINE PROTEASE INHIBITORS申请人:Moore Joel D.公开号:US20090123425A1公开(公告)日:2009-05-14The present invention relates to compounds of Formula I, II, or III, or pharmaceutically acceptable salts, esters, or prodrugs thereof: which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising a compound of the present invention.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
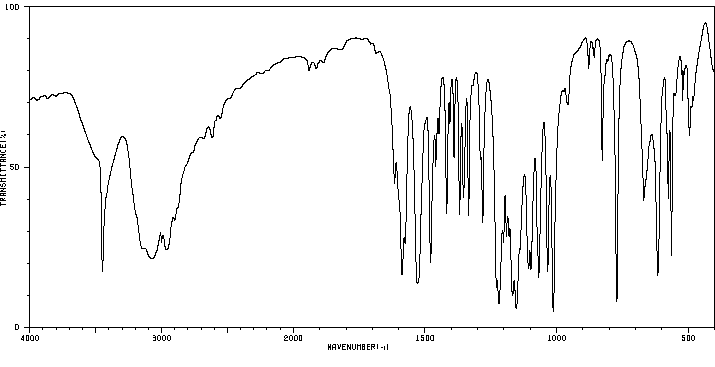
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮