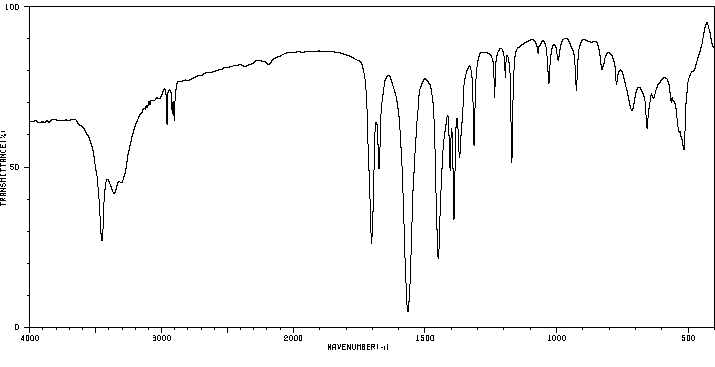
代谢
Although calcium levulinate dissociates into absorbable calcium ion once it is administered into the body, there have been studies to suggest that the levulinate component is metabolized to 4-hydroxypentanoate - a compound that has similar pharmacologic effects as but at a lower level of potency than the 'date rape' drug gamma-hydroxybutyrate.
来源:DrugBank