(3S,6R)-3-methyl-6-(1-methylethyl)cyclohexene | 5056-00-8
中文名称
——
中文别名
——
英文名称
(3S,6R)-3-methyl-6-(1-methylethyl)cyclohexene
英文别名
(+)-trans-p-menth-2-ene;(-)trans-p-menth-2-ene;(+)-trans-p-menthene;(1R,4R)-2-menthene;trans-2-p-menthene;cis-menth-2-ene;trans-p-Menth-2-ene;(3S,6R)-3-methyl-6-propan-2-ylcyclohexene
CAS
5056-00-8
化学式
C10H18
mdl
——
分子量
138.253
InChiKey
WHNGPXQYYRWQAS-NXEZZACHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:165.2±7.0 °C(Predicted)
-
密度:0.804±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— trans-2-p-Menthen-7-ol 19898-86-3 C10H18O 154.252
反应信息
-
作为反应物:描述:(3S,6R)-3-methyl-6-(1-methylethyl)cyclohexene 在 sodium periodate 、 间氯过氧苯甲酸 、 potassium hydroxide 作用下, 生成 2-isopropyl-5-methyladipaldehyde参考文献:名称:Acid-catalyzed aldol-Meerwein–Ponndorf–Verley-etherification reactions—access to defined configured quaternary stereogenic centers摘要:A novel asymmetric aldol-reduction-etherification process of aliphatic enolizable aldehydes is described. The intermediately formed aldol adducts-beta-hydroxyaldehydes-were reduced and transformed into the corresponding 1,3-diol ethers by external secondary alcohols at the same time. Thus, with the help of chiral secondary alcohols an access to optically active 1,3-diol ether is given. Furthermore, asymmetric cross-aldol-Meerwein-Ponndorf reactions of enolizable aldehydes can also be realized under these reaction conditions. Crown Copyright (C) 2011 Published by Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2011.11.069
-
作为产物:描述:在 Li2Cu3Me5 作用下, 以 乙醚 为溶剂, 反应 15.0h, 以81%的产率得到(3S,6R)-3-methyl-6-(1-methylethyl)cyclohexene参考文献:名称:顺式和反式3-甲基-6-甲基乙基环己烯和3-甲基-4-甲基乙基环己烯的区域和立体定向合成。烯丙基乙酸酯和氨基甲酸酯与Li 2 Cu 3 Me 5和LiCuMe 2的反应摘要:异构烯烃1-4是通过用Li 2 Cu 3 Me 5对适当的烯丙基氨基甲酸酯进行γ合成,γ取代而获得的。氨基甲酸酯的行为深受铜试剂性质的影响。DOI:10.1016/s0040-4039(00)87541-3
文献信息
-
Copper-Mediated and -Catalyzedo-DPPB-Directed Allylic Substitution作者:Bernhard Breit、Peter DemelDOI:10.1002/1615-4169(200107)343:5<429::aid-adsc429>3.0.co;2-k日期:2001.7Complete control of chemo-, regio- and stereoselectivity in the course of copper-catalyzed and -mediated allylic substitution could be obtained with the ortho-diphenylphosphanyl (o-DPPB) function as a reagent-directing leaving group. Complete chirality transfer by way of a syn-addition process has been achieved for cyclic and acyclic systems. Readily available Grignard reagents may be employed as nucleophiles
-
Practical Synthesis of Optically Pure Menthylamines Starting from Racemic Neomenthol作者:Siegfried Waldvogel、Nina WelschoffDOI:10.1055/s-0030-1258295日期:2010.11scalable route to racemic and highly enantiomerically enriched menthylamines exploits the technical product rac-neomenthol as the starting material. The elaborated protocol is based on nucleophilic substitution of the hydroxy moiety by azide. Subsequent reduction and resolution with tartaric acid provides the desired optically enriched menthylamines. amines - azides - chiral resolution - reduction - terpenoids
-
o-DPPB-Directed Copper-Mediated and -Catalyzed Allylic Substitution with Grignard Reagents作者:Peter Demel、Manfred Keller、Bernhard BreitDOI:10.1002/chem.200600225日期:2006.8.25explored as a directing leaving group in copper-mediated and copper-catalyzed allylic substitution with Grignard reagents. Complete control of chemo-, regio- and stereoselectivity with complete syn-1,3-chirality transfer was observed as a result of the directed nature of the reaction. No excess of organometallic reagent is required and the directing group can be recovered quantitatively. Coordination
-
Synthesis and Sensory Studies of Umami-Active Scaffolds作者:Michael Backes、Susanne Paetz、Tobias Vössing、Jakob Peter LeyDOI:10.1002/cbdv.201400113日期:2014.11molecules. A scalable synthesis of this challenging scaffold and new sensory insights will be presented. Interestingly, the umami characteristics differ remarkably, depending on constitutional and stereochemical features of the parent scaffold. During our studies, we could identify the carboxamide moiety as a crucial factor to influence the umami intensity of these scaffolds. In addition, the configuration2-异丙基-5-甲基双环[4.1.0]庚烷-7-甲酰胺类,1-4,已被鉴定为有效的鲜味分子。将介绍这种具有挑战性的支架和新的感官洞察力的可扩展合成。有趣的是,鲜味特征显着不同,这取决于母体支架的构成和立体化学特征。在我们的研究中,我们可以确定羧酰胺部分是影响这些支架鲜味强度的关键因素。此外,环丙基部分的构型会产生一些影响,而薄荷基支架的绝对构型,至少是测试的 D 和 L 构型,则不太重要。
-
Optical Rotatory Dispersion and Circular Dichroism of the Osmate Esters of Cyclic Mono-olefins作者:Naokazu Sakota、Shunsaku TanakaDOI:10.1246/bcsj.44.485日期:1971.2The osmate esters (as the di-pyridine adducts) of optically active mono-, di- and tricyclic mono-unsaturated terpenes were prepared for measurements of optical rotatory dispersion (ORD) and circular dichroism (CD). All the osmate esters of the cyclic olefins examined exhibited a strong Cotton effect in 470 mμ regions and a weak Cotton effect near 600 mμ regions, which were similar to those of the osmate
表征谱图
-
氢谱1HNMR
-
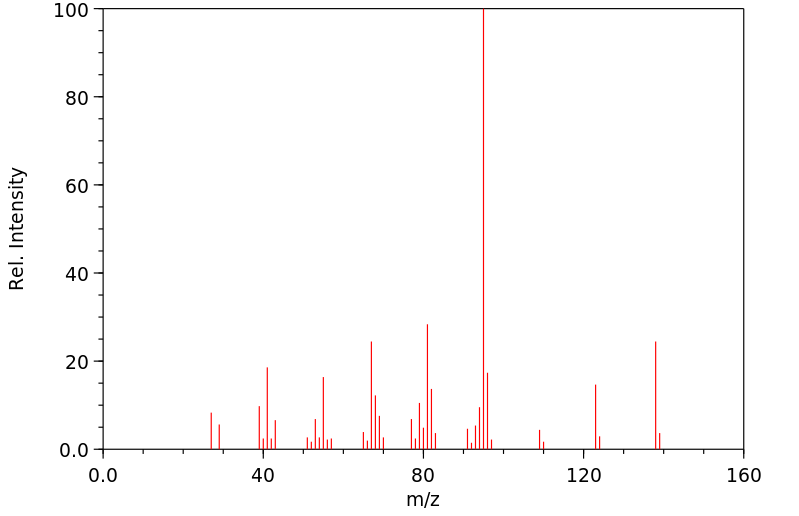
质谱MS
-
碳谱13CNMR
-
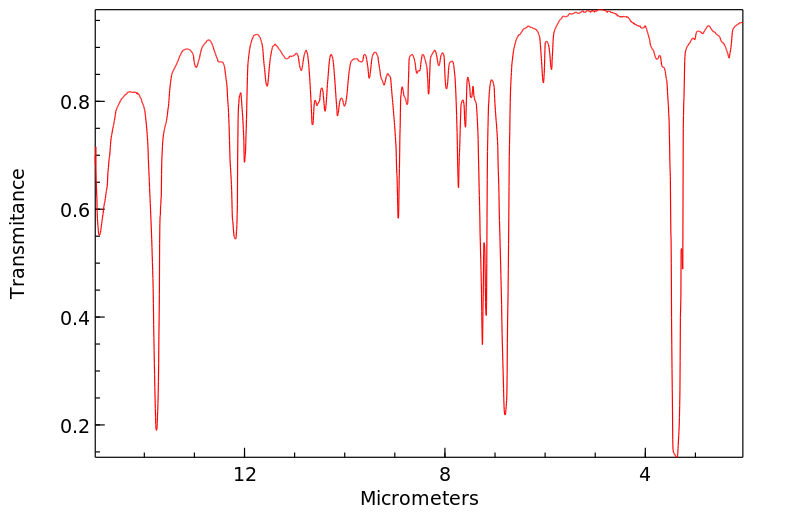
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸