trimethyl-5-methyl-2-(1-methylethylphenoxysilane) | 55012-80-1
中文名称
——
中文别名
——
英文名称
trimethyl-5-methyl-2-(1-methylethylphenoxysilane)
英文别名
(5-methyl-2-i-propylphenoxy)trimethylsilane;2-isopropyl-5-methylphenoxytrimethylsilane;trimethylsilyl ether of thymol;thymol-TMS;trimethyl-(5-methyl-2-propan-2-ylphenoxy)silane
CAS
55012-80-1
化学式
C13H22OSi
mdl
——
分子量
222.403
InChiKey
UTGMDFONUNJYQP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:255.2±19.0 °C(Predicted)
-
密度:0.894±0.06 g/cm3(Predicted)
-
保留指数:1312;1310;1310;1301;1312
计算性质
-
辛醇/水分配系数(LogP):4.33
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.54
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2931900090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 百里酚 5-methyl-2-(1-methylethyl)phenol 89-83-8 C10H14O 150.221
反应信息
-
作为反应物:描述:2,4-diferrocenyl-1,3-dithiadiphosphetane-2,4-disulfide 、 trimethyl-5-methyl-2-(1-methylethylphenoxysilane) 以 苯 为溶剂, 反应 2.0h, 以44%的产率得到O-(2-isopropyl-5-methylphenyl) S-trimethylsilyl (ferrocenyl)dithiophosphonate参考文献:名称:Dithiophosphorylation of trimethylsilyl ethers of carvacrol and thymol摘要:Carvacrol trimethylsilyl ether when reacting with P4S10 forms S-trimethylsilyl ester of O,O-bis(5-isopropyl-2-methylphenyl)dithiophosphoric acid. The reactions of 2,4-diorganyl-1,3,2,4-dithiadiphosphetane-2,4-disulfides with carvacrol (thymol) trimethylsilyl ether yield the S-trimethylsilyl esters of the corresponding dithiophosphoric acids.DOI:10.1134/s1070363216030117
-
作为产物:参考文献:名称:LAUTERBUCH, MANFRED;JUGER, GUNTER;JANCKE, HARALD;LEHMANN, ANDREAS;ZIMMERM+, CHEM. TECHN. , 42,(1990) N, C. 264-268摘要:DOI:
文献信息
-
Highly atom economical uncatalysed and I2-catalysed silylation of phenols, alcohols and carbohydrates, using HMDS under solvent-free reaction conditions (SFRC)作者:Marjan JerebDOI:10.1016/j.tet.2012.03.040日期:2012.5An uncatalysed silylation of phenols, regardless on the aggregate state and nature of the substituents with 0.55 equiv of HMDS under solvent-free reaction conditions (SFRC) at room temperature is reported. Sterically hindered phenols, carbohydrates and most of the alcohols additionally required a catalytic amount (up to 2 mol%) of iodine. The reaction protocol is very simple; obtaining a pure product, particularly of uncatalysed reactions, was frequently a completely solvent-free process. (C) 2012 Elsevier Ltd. All rights reserved.
-
Mishra; Singh, Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical, 2001, vol. 40, # 7, p. 772 - 774作者:Mishra、SinghDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
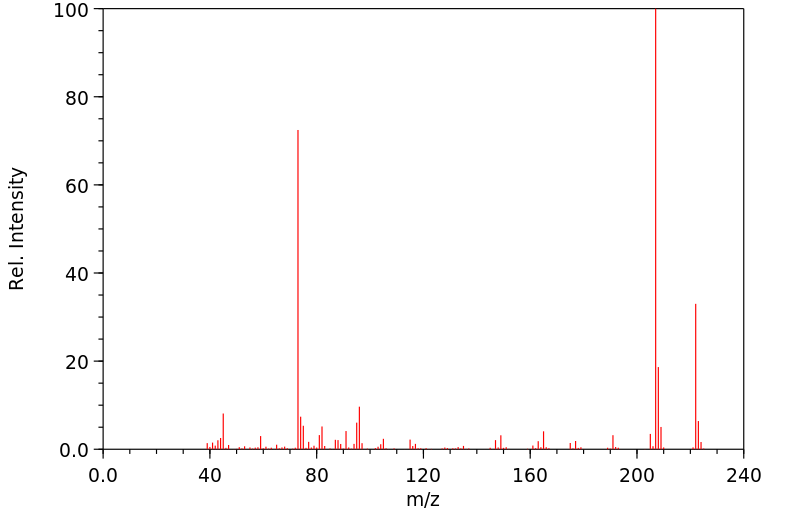
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸