1-(2-pyridyl)-3-(4-tolyl)propen-1-one | 24582-66-9
中文名称
——
中文别名
——
英文名称
1-(2-pyridyl)-3-(4-tolyl)propen-1-one
英文别名
1-(pyridin-2-yl)-3-p-tolylprop-2-en-1-one;3-(4-methylphenyl)-1-(2-pyridyl)-2-propen-1-one;1-pyridin-2-yl-3-p-tolyl-propenone;2-Propen-1-one, 3-(4-methylphenyl)-1-(2-pyridinyl)-, (2E)-;3-(4-methylphenyl)-1-pyridin-2-ylprop-2-en-1-one
CAS
24582-66-9
化学式
C15H13NO
mdl
——
分子量
223.274
InChiKey
QAXZVTXDKRUVTD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:384.9±34.0 °C(Predicted)
-
密度:1.120±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:30
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙酰基吡啶 2-acetylpyridine 1122-62-9 C7H7NO 121.139
反应信息
-
作为反应物:描述:1-(2-pyridyl)-3-(4-tolyl)propen-1-one 在 N-溴代丁二酰亚胺(NBS) 、 potassium tert-butylate 、 ammonium acetate 、 sodium hydroxide 作用下, 以 三甘醇二甲醚 、 乙醇 、 苯 为溶剂, 反应 0.58h, 生成 10-ethyl-3-[4-(2,2':6',2''-terpyridinyl-4'-yl)styryl]phenothiazine参考文献:名称:基于新型2,2':6',2''-吡啶的配体及其配合物的异构化和光物理性质的研究†摘要:一种新型的2,2':6',2''-基于吡啶的配体L及其配合物[ML 2 ](ClO 4)2 ·CH 2 Cl 2(M = Cd 1,Zn 2,Cu 4,Mn 5),合成[CoL 2 ](ClO 4)2 3,CdLI 2 6和CdL(SCN)2 7并进行充分表征。1–6的晶体结构通过单晶X射线衍射分析解决。系统地研究了所有配合物的线性吸收和发射性质以及三阶非线性光学性质。所述的平衡反式和-顺式-异构体的大号进行了实验和理论研究两者。配合物的构型和光物理性质对金属离子和阴离子的选择表现出很大的依赖性。DOI:10.1039/c1dt10752f
-
作为产物:描述:参考文献:名称:基于新型2,2':6',2''-吡啶的配体及其配合物的异构化和光物理性质的研究†摘要:一种新型的2,2':6',2''-基于吡啶的配体L及其配合物[ML 2 ](ClO 4)2 ·CH 2 Cl 2(M = Cd 1,Zn 2,Cu 4,Mn 5),合成[CoL 2 ](ClO 4)2 3,CdLI 2 6和CdL(SCN)2 7并进行充分表征。1–6的晶体结构通过单晶X射线衍射分析解决。系统地研究了所有配合物的线性吸收和发射性质以及三阶非线性光学性质。所述的平衡反式和-顺式-异构体的大号进行了实验和理论研究两者。配合物的构型和光物理性质对金属离子和阴离子的选择表现出很大的依赖性。DOI:10.1039/c1dt10752f
文献信息
-
PCN Pincer Palladium(II) Complex Catalyzed Enantioselective Hydrophosphination of Enones: Synthesis of Pyridine-Functionalized Chiral Phosphine Oxides as NC<sub>sp<sup>3</sup></sub>O Pincer Preligands作者:Xin-Qi Hao、Juan-Juan Huang、Tao Wang、Jing Lv、Jun-Fang Gong、Mao-Ping SongDOI:10.1021/jo5015307日期:2014.10.17A series of chiral PCN pincer Pd(II) complexes VI–XIII with aryl-based aminophosphine–imidazoline or phosphinite–imidazoline ligands were synthesized and characterized. They were examined as enantioselective catalysts for the hydrophosphination of enones. Among them, complex IX, which features a Ph2PO donor as well as an imidazoline donor with (4S)-phenyl and N-Tol-p groups, was found to be the optimal合成并表征了一系列具有芳基氨基膦-咪唑啉或次膦酸酯-咪唑啉配体的手性PCN夹心Pd(II)配合物VI - XIII。他们被检查为烯酮氢磷酸化的对映选择性催化剂。其中,具有Ph 2 PO供体以及具有(4S)-苯基和N- Tol- p基团的咪唑啉供体的配合物IX被认为是最佳催化剂。因此,在2–5 mol%的络合物IX存在下各种各样的烯酮与二芳基膦平稳地反应,以高收率得到对映体选择性高达98%ee的相应手性膦衍生物。尤其是,对于Pd中心具有强配位能力的杂芳基物质,例如含2-噻吩基,2-呋喃基和2-吡啶基的烯酮,也是当前催化体系的合适底物。例如,用二苯基膦将2-烯酰基吡啶进行氢磷酸化,然后用H 2 O 2氧化以高收率得到具有良好或优异的对映选择性的相应的吡啶官能化的手性氧化膦(10个实施例,至多95%ee)。此外,已经证明,所获得的含吡啶的氧化膦在与PdCl 2的反应中作为三齿配体,通过C sp 3
-
Comparative study of the vicinal functionalization of olefins with 2:1 bromide/bromate and iodide/iodate reagents作者:Manoj K. Agrawal、Subbarayappa Adimurthy、Bishwajit Ganguly、Pushpito K. GhoshDOI:10.1016/j.tet.2009.01.095日期:2009.4halohydrins, halo methyl ethers, and halo acetates from olefins using 2:1 Br−/BrO3− and I−/IO3− reagents. In many cases both reagents afforded products selectively in high yields. The highest halogen atom efficiencies attained were 97% and 93% for Br−/BrO3− and I−/IO3−, respectively. Of the two reagents, I−/IO3− was established to be the preferred reagent for vicinal functionalization of linear alkenes and
-
Tailor-made synthesis of fully alkylated/arylated nicotinates by FeCl<sub>3</sub>-mediated condensation of enamino esters with enones作者:Sho Hirai、Yurie Horikawa、Haruyasu Asahara、Nagatoshi NishiwakiDOI:10.1039/c7cc00051k日期:——A new method for synthesizing polyalkylated/arylated nicotinates is established using a condensation of enamino esters with enones in the presence of FeCl3. This method facilitates the introduction of alkyl or...在FeCl3存在下,使用烯胺酯与烯酮的缩合,建立了一种合成多烷基化/芳基烟酸酯的新方法。这种方法有助于引入烷基或...
-
Solvent-free bromination reactions with sodium bromide and oxone promoted by mechanical milling作者:Guan-Wu Wang、Jie GaoDOI:10.1039/c2gc16606b日期:——New solvent-free brominations of 1,3-dicarbonyl compounds, phenols, various alkenes including chalcones, azachalcones, 4-phenylbut-3-en-2-one, methyl cinnamate, styrene and 1,3-cyclohexadiene were efficiently achieved by employing sodium bromide and oxone under mechanical milling conditions. The brominated products were obtained in good to excellent yields.
-
Light‐Driven Enantioselective Synthesis of Pyrroline Derivatives by a Radical/Polar Cascade Reaction作者:Ricardo I. Rodríguez、Leonardo Mollari、José AlemánDOI:10.1002/anie.202013020日期:2021.2.23functionalization of acyl heterocycles through a hydrogen‐atom transfer (HAT) process and the use of tailor‐made ketimines as reliable electrophilic partners. This transformation is translated into an enantiomerically controlled radical/polar cascade reaction in which water is produced as the sole by‐product and stereoselectivity is dictated by coordination to a chiral‐at‐rhodium catalyst.
表征谱图
-
氢谱1HNMR
-
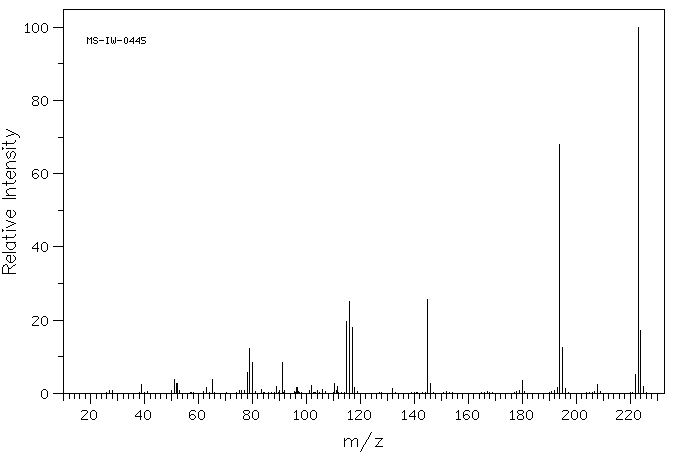
质谱MS
-
碳谱13CNMR
-
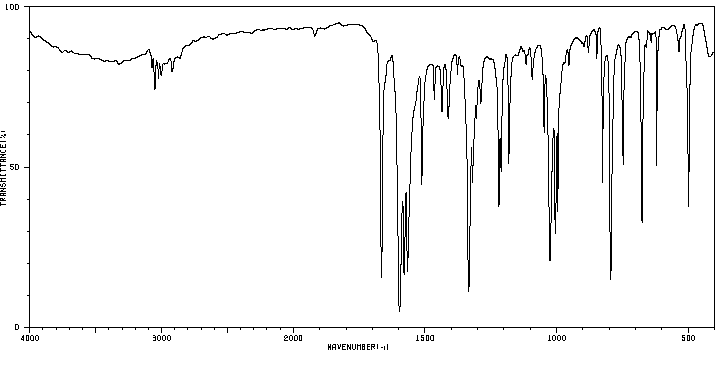
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30