代谢
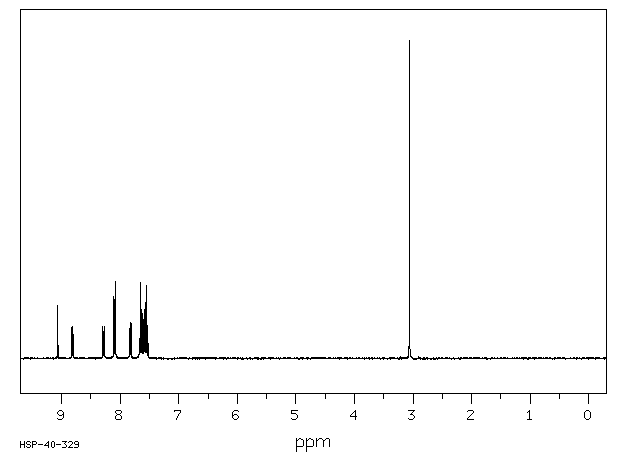
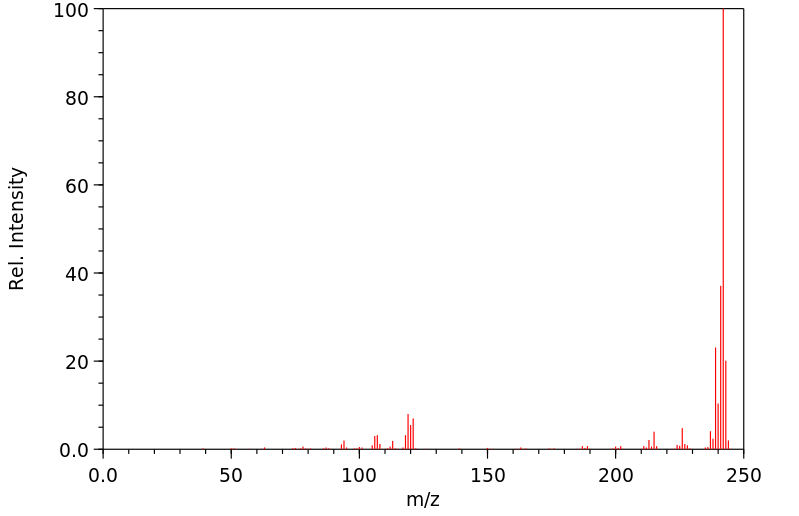
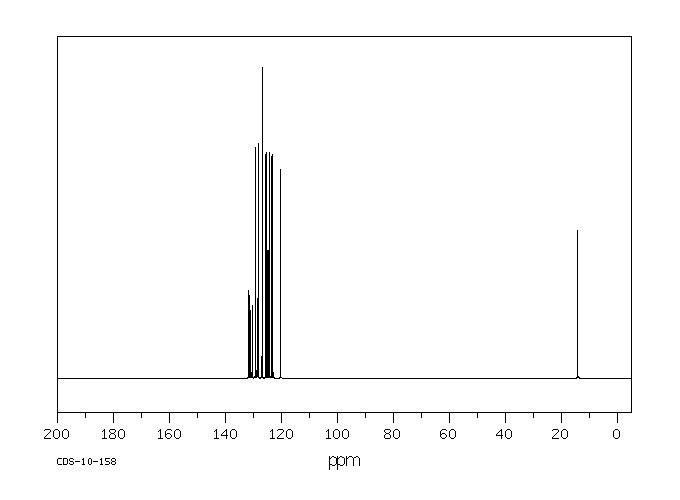
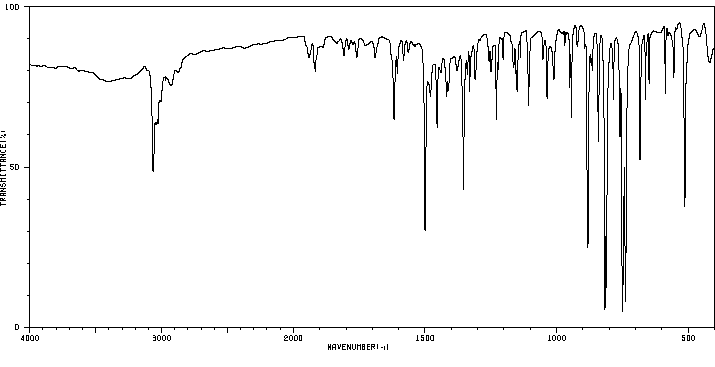
真菌对7-甲基苯[a]蒽(7-MBA)和7-羟基甲基苯[a]蒽(7-OHMBA)的代谢进行了研究。7-MBA被 Cunninghamella elegans 代谢,形成7-OHMBA-反式-8,9-二氢二醇和7-OHMBA-反式-3,4-二氢二醇作为主要代谢物。其他代谢物被鉴定为7-OHMBA、7-MBA-反式-8,9-二氢二醇和7-MBA-反式-3,4-二氢二醇,以及7-MBA-8,9,10,11-四醇。将7-OHMBA与C. elegans细胞一起培养表明,7-OHMBA-反式-8,9-二氢二醇和7-OHMBA-反式-3,4-二氢二醇是主要代谢物。经3-甲基胆蒽处理的鼠肝微粒体对7-MBA的代谢表明,代谢物在性质上与C. elegans形成的相似,只是在5,6和10,11位置形成了额外的二氢二醇代谢物。通过高效液相色谱法分离代谢物,并通过与参考化合物的色谱、紫外-可见吸收和质谱特性进行比较来鉴定它们。
The fungal metabolism of 7-methylbenz[a]anthracene (7-MBA) and 7-hydroxymethylbenz[a]anthracene (7-OHMBA) was studied. 7-MBA was metabolized by Cunninghamella elegans to form 7-OHMBA-trans-8,9-dihydrodiol and 7-OHMBA-trans-3,4-dihydrodiol as the predominant metabolites. Other metabolites were identified as 7-OHMBA, 7-MBA-trans-8,9-dihydrodiol and 7-MBA-trans-3,4-dihydrodiol, and 7-MBA-8,9,10,11-tetraol. Incubation of 7-OHMBA with C. elegans cells indicated that 7-OHMBA-trans-8,9-dihydrodiol and 7-OHMBA-trans-3,4-dihydrodiol were major metabolites. The metabolism of 7-MBA by rat liver microsomes from 3-methylcholanthrene-treated rats showed that the metabolites were qualitatively similar to those formed by C. elegans, except additional dihydrodiol metabolites were formed at the 5,6 and 10,11 positions. The metabolites formed were isolated by high-performance liquid chromatography and identified by comparing their chromatographic, UV-visible absorption and mass spectral properties with those of reference compounds.
来源:Hazardous Substances Data Bank (HSDB)