D-(+)-甘油醛 | 453-17-8
物质功能分类
中文名称
D-(+)-甘油醛
中文别名
R-(+)-甘油醛;D(+)-2,3二羟基丙醛;D(+)-甘油醛
英文名称
D-Glyceraldehyde
英文别名
(2R)-2,3-dihydroxypropanal
CAS
453-17-8
化学式
C3H6O3
mdl
MFCD00064378
分子量
90.0788
InChiKey
MNQZXJOMYWMBOU-VKHMYHEASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127-129 C
-
比旋光度:D25 +8.7° (c = 2 in H2O); D15 +21.2° (c = 18)
-
沸点:bp17 127-129°; bp10 123-126°
-
密度:1.272±0.06 g/cm3(Predicted)
-
闪点:112 °C
-
溶解度:可溶于DMSO、甲醇(微溶)、水(微溶)
计算性质
-
辛醇/水分配系数(LogP):-1.6
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S24/25,S26,S27,S36/37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2912491000
-
包装等级:I; II; III
-
储存条件:2-8°C
SDS
1.1 产品标识符
: D-(+)-甘油醛
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C3H6O3
分子式
: 90.08 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
建议的贮存温度: 2 - 8 °C
充气保存 对空气敏感。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粘稠液体
颜色: 淡棕
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂, 强还原剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: D-(+)-甘油醛
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C3H6O3
分子式
: 90.08 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
建议的贮存温度: 2 - 8 °C
充气保存 对空气敏感。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粘稠液体
颜色: 淡棕
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂, 强还原剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:D-(+)-甘油醛 在 rabbit 3-hydroxyhexobarbital dehydrogenase (AKR1C29) 、 还原型辅酶II(NADPH)四钠盐 作用下, 以 aq. phosphate buffer 、 乙酸乙酯 为溶剂, 反应 0.5h, 生成 甘油参考文献:名称:兔3-羟基己糖巴比妥脱氢酶是一种NADPH优先的还原酶,对酮类固醇,前列腺素D(2)以及其他内源性和异源性羰基化合物具有广泛的底物特异性。摘要:3-羟基己异巴比妥脱氢酶(3HBD)催化将NAD(P)(+)链接的3-羟基己异巴比妥氧化为3-羟基己异巴比妥。该酶被认为是异生物醇和某些羟基类固醇的脱氢酶,但其生理功能仍然未知。我们已经纯化了兔3HBD,分离了其cDNA,并检查了其对辅酶和底物的特异性,反应方向性和组织分布。3HBD是醛酮还原酶(AKR)超家族的成员(AKR1C29),并且在7.4的生理pH值下,NADP(H)优于NAD(H)。在与NADPH相关的还原反应中,3HBD对多种醌,酮和醛(包括3-,17-和20-酮类固醇和前列腺素D(2))显示出广泛的底物特异性,它们被转化为3alpha-,17beta-和20alpha -羟基类固醇和9alpha,11beta-前列腺素F(2),分别。特别是,α-二酮(如isatin和diacetyl)和脂质过氧化衍生的醛(如4-oxo-和4-hydroxy-2-nonenals)是显示低K(m)值(0DOI:10.1016/j.bcp.2013.08.024
-
作为产物:描述:参考文献:名称:PREPARATION OF
D -ANDL -GLYCERALDEHYDE FROM KETOHEXOSES摘要:不可用DOI:10.1139/v56-008
文献信息
-
Borate as a Phosphate Ester Mimic in Aldolase-Catalyzed Reactions: Practical Synthesis ofL-Fructose andL-Iminocyclitols作者:Masakazu Sugiyama、Zhangyong Hong、Lisa J. Whalen、William A. Greenberg、Chi-Huey WongDOI:10.1002/adsc.200600356日期:2006.12Dihydroxyacetone phosphate (DHAP)-dependent aldolases have been widely used for the organic synthesis of unnatural sugars or derivatives. The practicality of using DHAP-dependent aldolases is limited by their strict substrate specificity and the high cost and instability of DHAP. Here we report that the DHAP-dependent aldolase L-rhamnulose 1-phosphate aldolase (RhaD) accepts dihydroxyacetone (DHA)
-
Converging conversion – using promiscuous biocatalysts for the cell-free synthesis of chemicals from heterogeneous biomass作者:Samuel Sutiono、André Pick、Volker SieberDOI:10.1039/d0gc04288a日期:——and 2-KG production from D-xylose and L-arabinose. Simple optimization and reaction engineering allowed us to obtain BDO and 2-KG titers of 18 g L−1 and 42 g L−1, with theoretical yields of >75% and >99%, respectively. One of the promiscuous enzymes identified together with auxiliary promiscuous enzymes was also suitable for stereoconvergent synthesis from a mixture of D-glucose and D-galactose, predominant已经提出了由木质纤维素生物质生产化学品的替代品。然而,生物质利用的一个固有挑战是底物的异质性,导致水解后存在混合糖。混合糖的发酵通常导致差的产量和多种副产物的产生,因此使随后的下游加工复杂化。因此,近年来已经开发了系统生物催化来应对这一挑战。在这项工作中,使用基于序列的发现方法,鉴定了几种具有广泛底物混杂的新型酶,这些酶是D-木糖和L转化的合适生物催化剂。-阿拉伯糖,植物生物量中半纤维素的两个主要成分。这些混杂酶使得D-木糖和L-阿拉伯糖能够同时进行生物转化,从而以最大的3 g L -1 h -1的产率和> 95%的产率产生1,4-丁二醇(BDO)。使用O 2作为辅因子循环的辅助底物,该模型系统进一步适应于由戊糖生产α-酮戊二酸(2-KG)的最大生产率,达到4.2 g L -1 h -1和99%的产率。为了验证我们系统的潜在适用性,我们尝试扩大D-木糖和L的BDO和2-KG产量-阿拉伯糖。
-
Synthesis and SAR of New 5-Phenyl-3-ureido-1,5-benzodiazepines as Cholecystokinin-B Receptor Antagonists作者:Antonella Ursini、Anna M. Capelli、Robin A. E. Carr、Paolo Cassarà、Mauro Corsi、Ornella Curcuruto、Giovanni Curotto、Michele Dal Cin、Silvia Davalli、Daniele Donati、Aldo Feriani、Harry Finch、Gabriella Finizia、Giovanni Gaviraghi、Marc Marien、Giorgio Pentassuglia、Stefano Polinelli、Emiliangelo Ratti、Aldo Reggiani、Giorgio Tarzia、Giovanna Tedesco、Maria E. Tranquillini、David G. Trist、Frank T. M. Van AmsterdamDOI:10.1021/jm990967h日期:2000.10.1A series of 5-phenyl-3-ureidobenzodiazepine-2,4-diones was synthesized and evaluated as cholecystokinin-B (CCK-B) receptor antagonists. Structure-activity relationship (SAR) studies revealed the importance of the N-1 substituent for potent and selective CCK-B affinity. Addition of substituents at the urea side chain provided in some cases more potent compounds. Moreover the introduction of bulky substituents
-
Combining the Petasis 3-Component Reaction with Multiple Modes of Cyclization: A Build/Couple/Pair Strategy for the Synthesis of Densely Functionalized Small Molecules作者:Thomas Flagstad、Mette R. Hansen、Sebastian T. Le Quement、Michael Givskov、Thomas E. NielsenDOI:10.1021/co500091f日期:2015.1.12strategy for the synthesis of complex and densely functionalized small molecules is presented. The strategy relies on synthetically tractable building blocks (build), that is, diversely substituted hydrazides, α-hydroxy aldehydes, and boronic acids, which undergo Petasis 3-component reactions (couple) to afford densely functionalized anti-hydrazido alcohols. The resulting scaffolds can subsequently
-
Synthesis of Cryptochiral (<i>R</i>,<i>R</i>)-2,3-Dideuterooxirane as Stereochemical Reference Compound and Chemical Correlation with D-(+)-Glyceraldehyde作者:Oliver Trapp、Kerstin ZawatzkyDOI:10.1002/ijch.201600111日期:2016.11correlated with sugars, amino acids, etc. is of great interest. Here, we present the synthesis of enantiopure (R,R)‐2,3‐dideuterooxirane, of which the absolute configuration has been unambiguously determined by foil‐induced Coulomb explosion imaging, and the correlation with the configuration of D‐(+)‐glyceraldehyde.
表征谱图
-
氢谱1HNMR
-
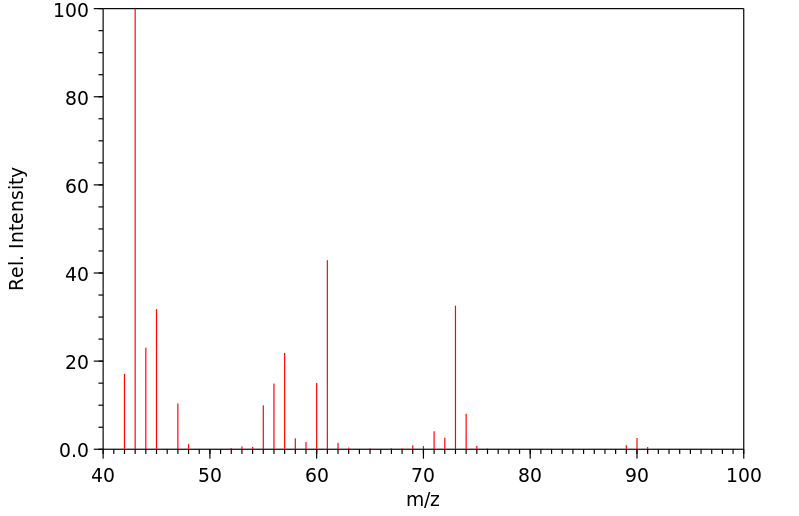
质谱MS
-
碳谱13CNMR
-
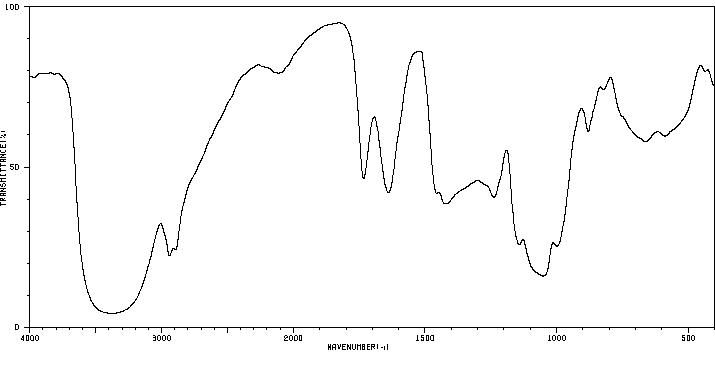
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷