草酸乙酯 | 1457-85-8
中文名称
草酸乙酯
中文别名
草酰苯胺乙酯
英文名称
Ethyl oxanilate
英文别名
oxo(phenylamino)-acetic acid, ethyl ester;ethyl 2-oxo-2-(phenylamino)acetate;ethyl 2-anilino-2-oxoacetate
CAS
1457-85-8
化学式
C10H11NO3
mdl
MFCD00009118
分子量
193.202
InChiKey
YDGAUBHNAKCSKF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:67-69 °C(lit.)
-
沸点:329.41°C (rough estimate)
-
密度:1.2307 (rough estimate)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:55.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2924299090
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P260,P264,P273,P301+P312,P305+P351+P338,P314
-
危险品运输编号:3077
-
危险性描述:H302,H319,H372,H410
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。同时,确保工作环境具备良好的通风或排气设施。
SDS
| Name: | Ethyl oxanilate 98% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1457-85-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1457-85-8 | Ethyl oxanilate | 98 | 215-945-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1457-85-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 67.00 - 69.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C10H11NO3
Molecular Weight: 193.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1457-85-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl oxanilate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 1457-85-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1457-85-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1457-85-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苯基氨基乙酸乙基醚 N-phenylglycine ethyl ester 2216-92-4 C10H13NO2 179.219 —— (methyl-phenyl-amino)-acetic acid ethyl ester 21911-74-0 C11H15NO2 193.246 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (4-溴苯胺基)氧代乙酸乙酯 ethyl N-p-bromophenyloxamate 24451-15-8 C10H10BrNO3 272.098 草酸一醯胺苯 oxanilic acid 500-72-1 C8H7NO3 165.148 —— ethyl N-(4-nitrophenyl)oxamate 5416-11-5 C10H10N2O5 238.2 N-苯基甘氨酸 N-Phenylglycine 103-01-5 C8H9NO2 151.165 —— ethyl 2-((2-nitrophenyl)amino)-2-oxoacetate 17293-40-2 C10H10N2O5 238.2 —— phenyl-oxalamide 10543-64-3 C8H8N2O2 164.164 —— 2-hydroxy-2-methylpropionanilide 2760-38-5 C10H13NO2 179.219
反应信息
-
作为反应物:参考文献:名称:2-(芳氨基)-2-(芳亚氨基)乙酰胺的合成摘要:2-(芳基氨基)-2-氧代乙酸酯与PCl5反应得到2-(芳基氨基)-2,2-二氯乙酸酯。这些化合物与苯胺衍生物的反应允许方便地合成多种 2-(芳基氨基)-2-(芳基亚氨基)乙酰胺。(© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2004)DOI:10.1002/ejoc.200300625
-
作为产物:描述:参考文献:名称:叔丁基过氧化氢促进缺电子烯胺的无金属氧化 C=C 键裂解摘要:描述了一种新型的叔丁基氢过氧化物 (TBHP) 促进的烯胺氧化 C=C 双键裂解。在 TBHP 存在下,将缺电子烯胺的氯苯溶液在 80°C 下加热两小时,导致 C=C 键断裂。本研究为利用 TBHP 形成 C=O 双键提供了一种新策略。DOI:10.1055/s-0036-1588990
文献信息
-
Certain Nitrogen Containing Bicyclic Chemical Entities for Treating Viral Infections申请人:Baskaran Subramanian公开号:US20100204265A1公开(公告)日:2010-08-12Provided are certain chemical entities, pharmaceutical compositions, and methods of treatment of a member of the flaviviradae family of viruses such as hepacivirus (Hepatitis C or HCV).提供了一些化学实体、药物组合物以及治疗黄病毒科家族成员,如肝病毒(丙型肝炎或HCV)的方法。
-
Copper-Catalysed (Diacetoxyiodo)benzene-Promoted Aerobic Esterification Reaction: Synthesis of Oxamates from Acetoacetamides作者:Zhiguo Zhang、Xiaolong Gao、Haifeng Yu、Guisheng Zhang、Jianming LiuDOI:10.1002/adsc.201800616日期:2018.9.3A copper‐catalysed (diacetoxyiodo)benzene‐promoted aerobic esterification reaction of acetoacetamides was developed for the synthesis of oxamates, which are useful precursors in synthetic organic chemistry. This practical and mild synthetic approach proceeded at 25 °C under open‐air conditions and afforded methyl 2‐oxo‐2‐(phenylamino)acetates in good to excellent yields combined with C−C σ‐bond cleavage
-
Preparations of carboxylic acid esters containing heptafluoroisopropyl groups作者:T. Suyama、S. Kato、Y. MizutaniDOI:10.1016/s0022-1139(00)80184-0日期:1992.1fluoroglyoxylic acid esters and fluoroformic acid esters with perfluoropropene (PFP) yielded perfluoro(3-methyl-2-oxobutyric) acid esters and perfluoroisobutyric acid esters, respectively. Oxamide derivatives and 2,3-quinoxalinediol have been prepared by the reaction of perfluoro(3-methyl-2-oxobutyric) acid esters with amines and o- phenylenediamine, respectively. Perfluoro(3-methyl-2-oxobutyric) acid esters
-
酰胺咪唑类衍生物及其用途
-
New Highlights in the Synthesis of 4-Aryl-1,4-dihydropyrazines作者:Jing-Yu He、Xiu-Qing Song、Hong Yan、Ru-Gang ZhongDOI:10.1002/jhet.989日期:2012.11The 4‐aryl‐1,4‐dihydropyrazines were prepared via the cyclization of N,N‐bisalkylated anilines with ammonium acetate. These reactions were aided by improvements in the synthesis of N,N‐bisalkylated anilines which were alkylated with anilines using ethyl 2‐diazo acetoacetate in a reaction catalyzed by rhodium acetate in the absence of oxygen. A possible mechanistic route is postulated on the basis of
表征谱图
-
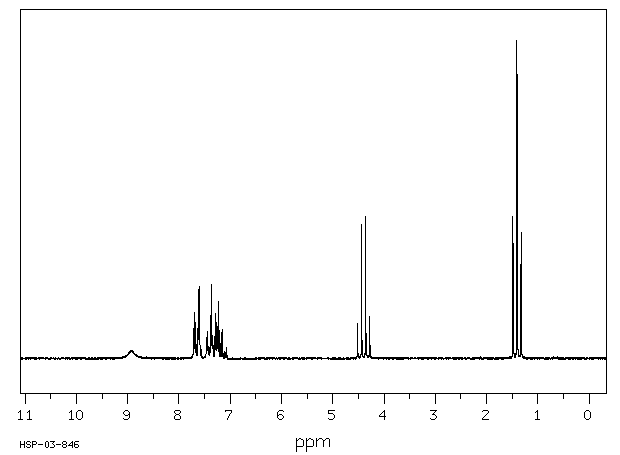
氢谱1HNMR
-
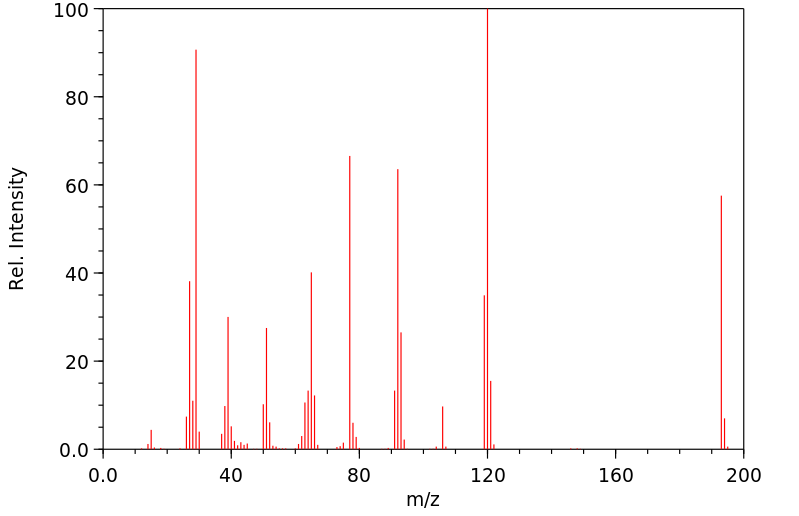
质谱MS
-
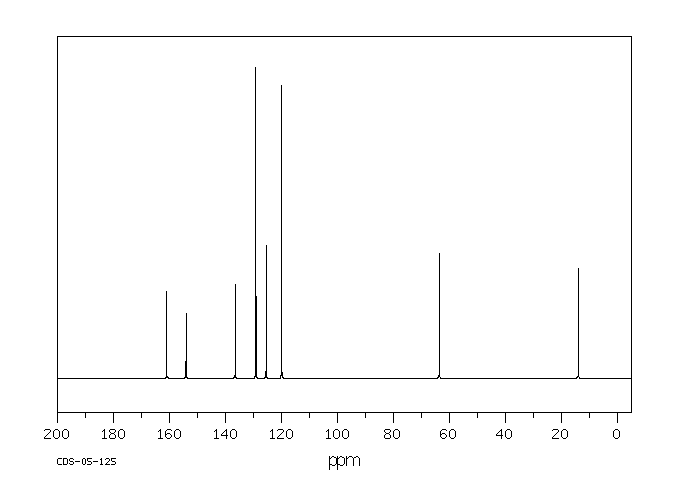
碳谱13CNMR
-
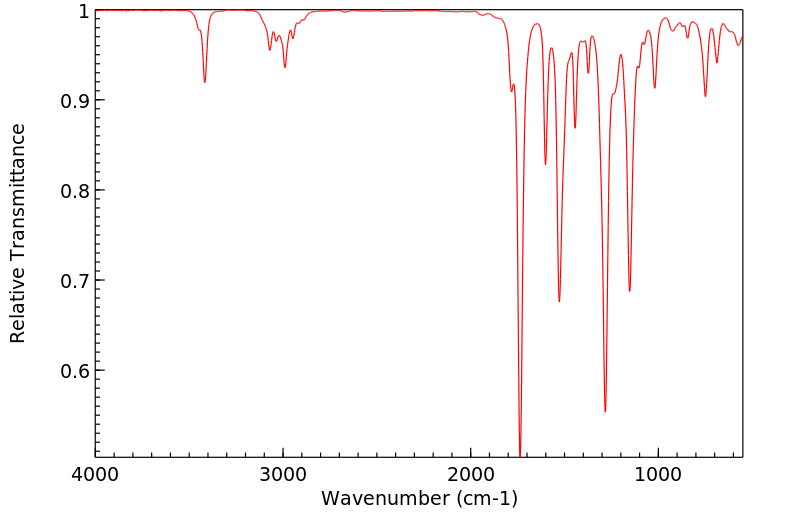
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸