反-2,2-二甲基-3-庚烯 | 19550-75-5
中文名称
反-2,2-二甲基-3-庚烯
中文别名
——
英文名称
trans-2,2-dimethyl-3-heptene
英文别名
(E)-2,2-dimethylhept-3-ene
CAS
19550-75-5
化学式
C9H18
mdl
——
分子量
126.242
InChiKey
BQOCYCICSYUPRF-BQYQJAHWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:135.0±7.0 °C(Predicted)
-
密度:0.72
-
闪点:19 °C
-
保留指数:802
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.78
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品运输编号:UN 3295
-
海关编码:2901299090
-
包装等级:II
-
危险类别:3
SDS
反应信息
-
作为反应物:描述:参考文献:名称:过氧化氢水溶液对碳二亚胺促进的烯烃的环氧化作用摘要:已经发现在含有过氧化氢的羟基溶剂中与温和的碱性或酸性催化剂一起可商购获得的碳二亚胺可促进烯烃的环氧化。市售的30%过氧化氢水溶液用作该方法的氧化剂。推测的反应性物质是通过向碳二亚胺中加入过氧化氢而在现场产生的过氧异脲。DOI:10.1021/jo972026n
-
作为产物:参考文献:名称:NMR rate study on the Wittig reaction of 2,2-dimethylpropanal and tributylbutylidenephosphorane摘要:DOI:10.1016/s0040-4039(00)99465-6
文献信息
-
Stereochemistry and mechanism of the Wittig reaction. Diasteromeric reaction intermediates and analysis of the reaction course作者:Bruce E. Maryanoff、Allen B. Reitz、Martin S. Mutter、Robert R. Whittle、R. A. OlofsonDOI:10.1021/ja00284a034日期:1986.11respectively). The above-mentioned rate studies, entailing low-temperature ' 8 , I3C, and/or "P NMR spectroscopic measurements, indicate that the equilibration of cisto truns-oxaphosphetane arises from a larger rate of reversion of the cis-oxaphosphetane to ylide and aldehyde. Crossover experiments involving deprotonation of diastereomerically pure P-hydroxyphosphonium salts 7a and Sa (R = Ph), followed by the通过在低温下使用高场 NMR 光谱在 Wittig 烯化反应中直接观察到单个非对映异构体 1,2-氧杂膦烷,从而可以对这些中间体进行详细评估。随着时间的推移监测苯甲醛与叶立德 4 和与叶立德 11 的反应,以获得 Wittig 反应的第一个亲密动力学视角。提供完整的动力学数据以及我们分析的细节。在许多情况下,不稳定的磷叶立德与醛的反应显示低温下顺式和反式氧杂膦烷的相对比例(在显着烯烃形成之前)与 Z 和 E 烯烃的最终比例之间不对应,导致夸大E 烯烃的生产(为方便起见,这种现象称为“立体化学漂移”)。因此,在这些反应中存在热力学控制的措施。对于三芳基叶立德 4,在锂盐的存在下,很容易与芳香醛苯甲醛发生平衡。发现向具有 4 和苯甲醛的更稳定的反式异构体(6a,R = Ph)的转变是浓度依赖性的,这可能是因为 THF 溶剂对锂阳离子的竞争性螯合。如果反式氧杂膦烷严重污染顺式异构体,则在没有锂的
-
Novel<i>ortho</i>-Alkoxy-Substituted Phosphorus Ylides and Their Stereoselectivity in Witting Reactions作者:Suruliappa Jeganathan、Masamitsu Tsukarmoto、Manfred SchlosserDOI:10.1055/s-1990-26801日期:——The stereochemistry of the reactions between tris(2-methoxy-methoxyphenyl)phosphonioethanide (1f), -butanide (2f), and -phenyl-methanide (3f) and a variety of aldehydes was investigated. Ylides having a β-unbranched aliphatic sidechain, such as 2f, and saturated straight-chain aldehydes give olefins with unprecedented cis-selectivity (cis/trans â 200:1).
-
Olefin Epoxidation with Bis(trimethylsilyl) Peroxide Catalyzed by Inorganic Oxorhenium Derivatives. Controlled Release of Hydrogen Peroxide作者:Andrei K. Yudin、Jay P. Chiang、Hans Adolfsson、Christophe CopéretDOI:10.1021/jo010369m日期:2001.6.1using bis(trimethylsilyl) peroxide (BTSP) as oxidant in place of aqueous H(2)O(2). Using a catalytic amount of a proton source, controlled release of hydrogen peroxide helps preserve sensitive peroxorhenium species and enables catalytic turnover to take place. Systematic investigation of the oxorhenium catalyst precursors, substrate scope, and effects of various additives on olefin epoxidation with
-
Synthesis of Cyclic Peroxides by Chemo- and Regioselective Peroxidation of Dienes with Co(II)/O<sub>2</sub>/Et<sub>3</sub>SiH作者:Takahiro Tokuyasu、Shigeki Kunikawa、Kevin J. McCullough、Araki Masuyama、Masatomo NojimaDOI:10.1021/jo048359j日期:2005.1.1Consistent with results from simple alkenes, the chemo- and regioselective peroxidation of dienes was also realized. Depending on the diene structure, the product included not only the expected acyclic unsaturated triethylsilyl peroxides but also 1,2-dioxolane and 1,2-dioxane derivatives via intramolecular cyclization of the unsaturated peroxy radical intermediates.
-
Homoallylic substitution reactions of lithium dialkyl cuprates with cyclopropylcarbinyl halides: mechanistic considerations作者:Robert T. Hrubiec、Michael B. SmithDOI:10.1016/s0040-4020(01)91792-2日期:1984.1kanes, , react to give good yields of the homoallylic substitution product, . Less reactive organocuprates react with to give mixtures of and the direct substitution product, . These results are consistent with a copper(I) radical intermediate which undergoes facile rearrangement prior to reductive coupling.
表征谱图
-
氢谱1HNMR
-
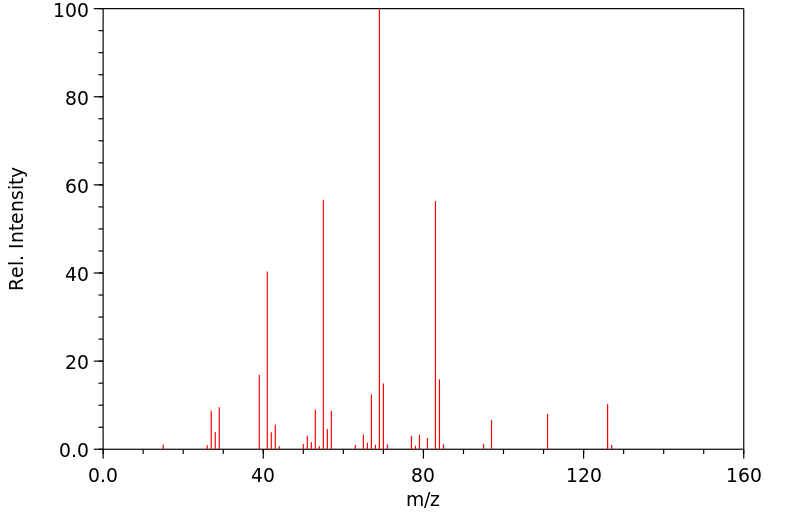
质谱MS
-
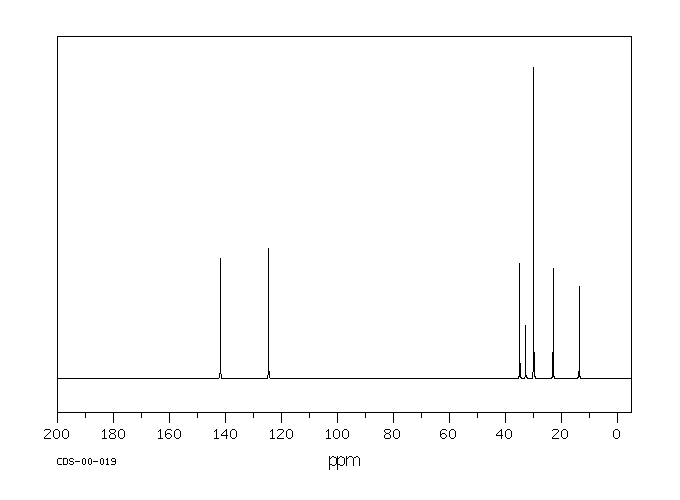
碳谱13CNMR
-
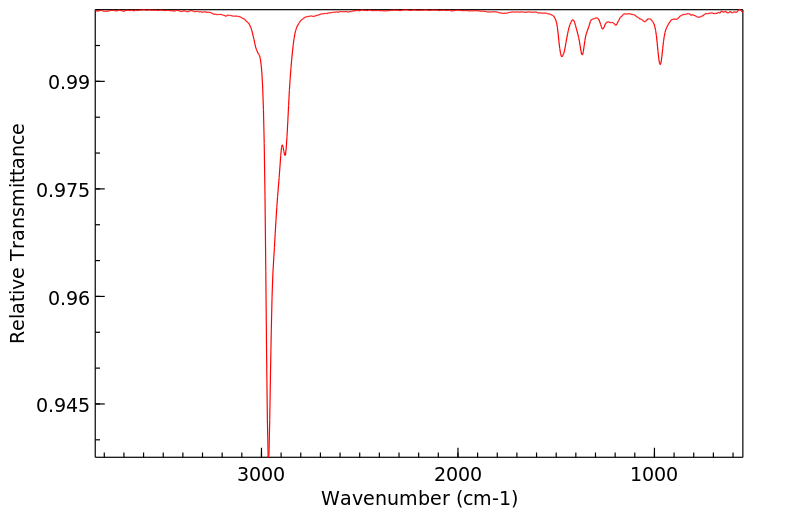
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-