5,9-十四碳二炔 | 51255-61-9
中文名称
5,9-十四碳二炔
中文别名
5,9-十四烷二炔
英文名称
5,9-tetradecadiyne
英文别名
tetradeca-5,9-diyne
CAS
51255-61-9
化学式
C14H22
mdl
MFCD00041652
分子量
190.329
InChiKey
MYMVMGJYNYKGGP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:132-134°C 12mm
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,且没有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):5.4
-
重原子数:14
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-溴庚-2-炔 1-bromo-2-heptyne 18495-26-6 C7H11Br 175.068
反应信息
-
作为反应物:描述:参考文献:名称:Garwood,R.F. et al., Journal of the Chemical Society. Perkin transactions I, 1973, p. 2714 - 2721摘要:DOI:
-
作为产物:参考文献:名称:Garwood,R.F. et al., Journal of the Chemical Society. Perkin transactions I, 1973, p. 2714 - 2721摘要:DOI:
文献信息
-
Cyclization of diacetylenes to E,E exocyclic dienes. Complementary procedures based on titanium and zirconium reagents作者:William A. Nugent、David L. Thorn、R. L. HarlowDOI:10.1021/ja00243a036日期:1987.4La cyclisation intramoleculaire de composes diyniques a ete effectuee en utilisant l'une des combinaisons de reactifs: Cp 2 TiCl 2 /PMePh 2 /Na(Hg) ou Cp 2 ZrCl 2 /Mg/HgCl 2La cyclisation intramoleculaire de composes diyniques a ete effectuee en utilisant l'une des combinaisons de reactifs: Cp 2 TiCl 2 /PMePh 2 /Na(Hg) ou Cp 2 ZrCl 2 /Mg/HgCl 2
-
Stereochemistry of mono-tetrahydrofuranyl moiety in cytotoxic polyketides. Part A: Synthesis of model compounds作者:Jonathan B. Gale、Jing-Guang Yu、Xiufeng E. Hu、Anakshi Khare、David K. Ho、John M. CassadyDOI:10.1016/s0040-4039(00)73795-6日期:1993.9Dimesitoate esters of α,α-dibutyl-2,5-tetrahydrofurandimethanols of different relative stereochemistry were prepared. They serve as model compounds for a 1H-NMR-based stereochemical analysis of the mono-tetrahydrofuranyl moiety of cytotoxic polyketides.
-
Kasatkin, A. N.; Kulak, A. N.; Tolstikov, G. A., Journal of Organic Chemistry USSR (English Translation), 1988, vol. 24, # 10, p. 1875 - 1889作者:Kasatkin, A. N.、Kulak, A. N.、Tolstikov, G. A.、Lomakina, S. I.DOI:——日期:——
-
Oxidative Cyclization of Diols Derived from 1,5-Dienes: Formation of Enantiopurecis-Tetrahydrofurans by Using Catalytic Osmium Tetroxide; Formal Synthesis of (+)-cis-Solamin作者:Timothy J. Donohoe、Sam ButterworthDOI:10.1002/anie.200500513日期:2005.7.25
-
Intramolekulare Cyclisierung von terminal disubstituierten α, ω-Diinen an Titanocen “Cp2Ti” mit einer nachfolgenden, ungewöhnlichen Cp-ringöffnung und neuen intramolekularen CC-Knüpfung作者:A. Tillack、W. Baumann、A. Ohff、C. Lefeber、A. Spannenberg、R. Kempe、U. RosenthalDOI:10.1016/0022-328x(96)06307-3日期:1996.8The reaction of Cp(2)Ti(Me(3)SiC(2)SiMe(3)) (1) with terminal disubstituted alpha,omega-diynes RC=C-(CH2)(n)-C=CR affords, after substitution of Me(3)SiC(2)SiMe(3), bicyclic titanacyclopentadienes via intramolecular cyclization. The stability of the obtained products 2, 3 and 5 is determined by the spacer length (n = 2, 4, 5, 6). The four-membered ring derivatives (n = 2) 2a and 2b were obtained in good yield. In the case of n = 4 the bicyclic six-membered ring 3 was formed at first, which rearranges to a stable tricyclic eta(4): eta(3)-dihydroindenyl-Ti complex 4 by Cp cleavage and intramolecular C-C coupling. Complex 4 was characterized by X-ray structure analysis and NMR spectroscopy. An increase of spacer length (n > 4) provides indefinable secondary and decomposition products.
表征谱图
-
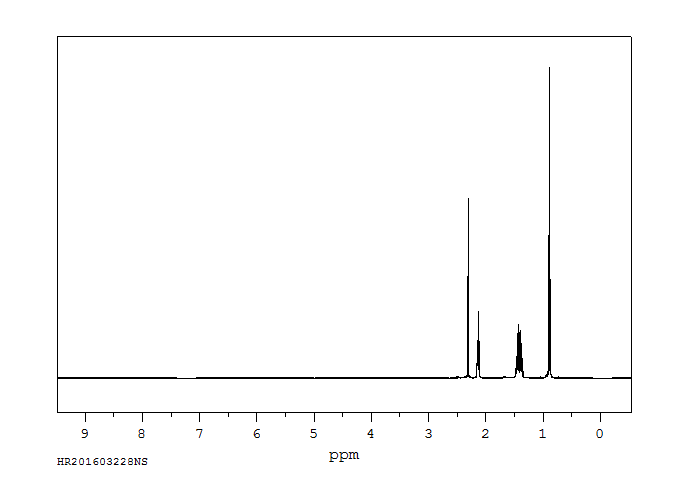
氢谱1HNMR
-
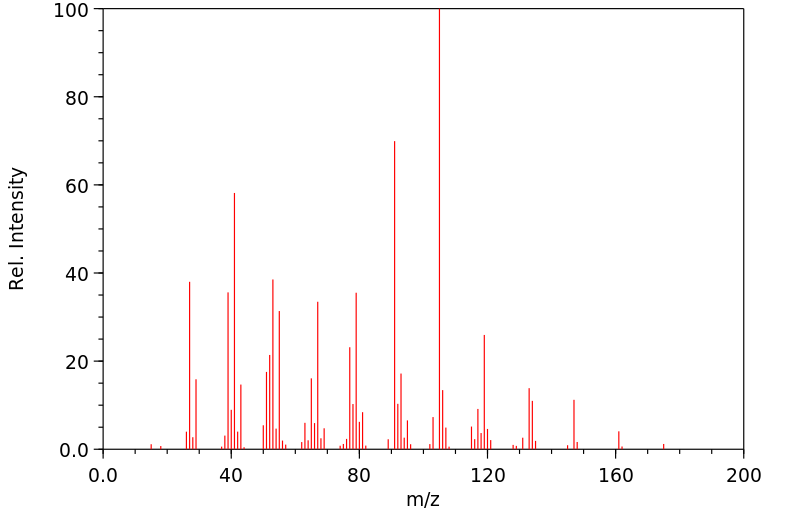
质谱MS
-
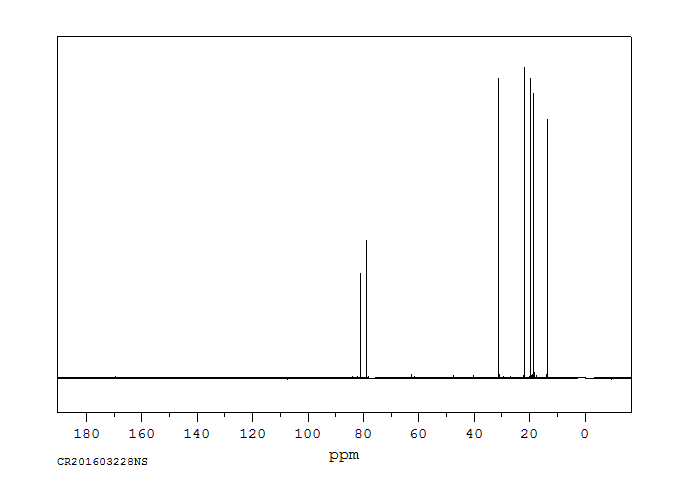
碳谱13CNMR
-
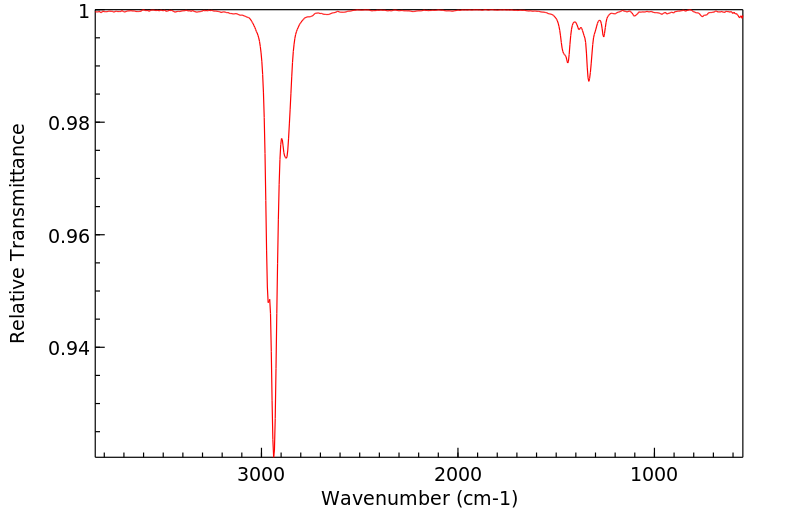
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-