8-甲氧基-2-苯基色原酮 | 26964-26-1
中文名称
8-甲氧基-2-苯基色原酮
中文别名
——
英文名称
8-methoxyflavone
英文别名
8-Methoxyflavon;8-methoxy-2-phenyl-4H-chromen-4-one;8-methoxy-2-phenylchromen-4-one
CAS
26964-26-1
化学式
C16H12O3
mdl
——
分子量
252.269
InChiKey
FOXRKQWVTGRONY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:19
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2914509090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and Biological Evaluation of Substituted Flavones as Gastroprotective Agents摘要:Flavone (1) was found to protect against ethanol-induced gastric damage in rats; however, it is known that certain compounds in the flavone class, including flavone itself, are inducers of hepatic drug metabolizing enzymes. With the hope of identifying gastroprotective flavones that have minimal effects on drug metabolizing enzymes, we have synthesized and evaluated selected flavone analogs. Gastroprotective potency in the ethanol model was retained by methoxy substitution in the 5-position (4) and by methoxy (12) or methyl (14) substitution in the 7-position. A number of substituted analogs of the potent molecule 5-methoxyflavone (4) were also synthesized, and in many cases, these substitutions provided gastroprotective molecules. In order to assess liver enzyme induction potential, two of the gastroprotective flavones, 7-methoxyflavone (12) and 5-methoxy-4'-fluoroflavone (26), were examined for their effect on liver microsomal cytochrome P450 and 7-ethoxyresorufin O-dealkylase (CYP1A) activity. These two compounds caused minimal changes in the cytochrome P450 concentration and were considerably less potent than beta-naphthoflavone as inducers of CYP1A enzyme activity. Furthermore, following oral administration to rats, 5-methoxy-4'-fluoroflavone (26) was found to protect against indomethacin-induced gastric damage. These results indicate that, through appropriate substitution, flavones can be obtained that are gastroprotective but have minimal effects on drug-metabolizing enzymes.DOI:10.1021/jm00025a011
-
作为产物:描述:参考文献:名称:Ahluwalia et al., Proceedings - Indian Academy of Sciences, Section A, 1953, # 38, p. 480,490摘要:DOI:
文献信息
-
Ru(<scp>ii</scp>)-Catalyzed C–H activation and annulation of salicylaldehydes with monosubstituted and disubstituted alkynes作者:Swagata Baruah、Partha Pratim Kaishap、Sanjib GogoiDOI:10.1039/c6cc07204f日期:——The Ru(II)-catalyzed C-H activation and annulation reaction of salicylaldehydes and disubstituted alkynes affords chromones in high yields. This reaction works with terminal alkynes also and tolerates wide range of sensitive...
-
Regioselective synthesis of flavone derivatives via DMAP-catalyzed cyclization of o-alkynoylphenols作者:Masahito Yoshida、Yuta Fujino、Koya Saito、Takayuki DoiDOI:10.1016/j.tet.2011.09.063日期:2011.12A catalytic amount of DMAP promoted cyclization of o-alkynoylphenols via a 6-endo cyclization mode leading to flavone derivatives in high yields without forming 5-exo cyclized aurone derivatives. Utilizing this method, methoxy substituted flavone and alkyl substituted γ-benzopyranone derivatives were synthesized.
-
Rhodium(III)-catalyzed one-pot synthesis of flavonoids from salicylaldehydes and sulfoxonium ylides作者:Kang Cheng、Jinkang Chen、Licheng Jin、Jian Zhou、Xinpeng Jiang、Chuanming YuDOI:10.1177/1747519819867230日期:2019.9Rh(III)-catalyzed C–H activation of salicylaldehyde followed by an insertion reaction with sulfoxonium ylides and cyclization is applied to the synthesis of flavonoids. This one-pot strategy exhibits good functional group tolerance and gives flavones in moderate-to-good yields.
-
Synthesis of 4<i>H</i>-Chromen-4-one Derivatives by Intramolecular Palladium-Catalyzed Acylation of Alkenyl Bromides with Aldehydes作者:Yixia Yue、Jinsong Peng、Deqiang Wang、Yunyun Bian、Peng Sun、Chunxia ChenDOI:10.1021/acs.joc.7b00640日期:2017.5.19The palladium-catalyzed intramolecular acylation of alkenyl bromides and aldehydes was developed for an efficient synthesis of 4H-chromen-4-ones. With Pd(PPh3)4/Xphos as the catalyst and K2CO3 as the base, this protocol was applied to synthesize a small library of diversely functionalized flavonoids in moderate to good yields in 1,4-dioxane.
-
Reagent-free intramolecular hydrofunctionalization: a regioselective 6-<i>endo-dig</i> cyclization of <i>o</i>-alkynoylphenols作者:Chanhyun Jung、Siyuan Li、Kwanghee Lee、Mayavan Viji、Heesoon Lee、Soonsil Hyun、Kiho Lee、Young Kee Kang、Chhabi Lal Chaudhary、Jae-Kyung JungDOI:10.1039/d1gc04848a日期:——Solvent-directed intramolecular hydrofunctionalization of readily available o-alkynoylphenols 1 was successfully achieved under reagent-free conditions. The hydrofunctionalization of 1 occurred by nucleophilic attack on the phenolic oxygen followed by consecutive migration of the phenolic H atom to the alkyne center, eventually affording γ-benzopyranones 2. The phenol O–H group forms intramolecular H-bonds
表征谱图
-
氢谱1HNMR
-
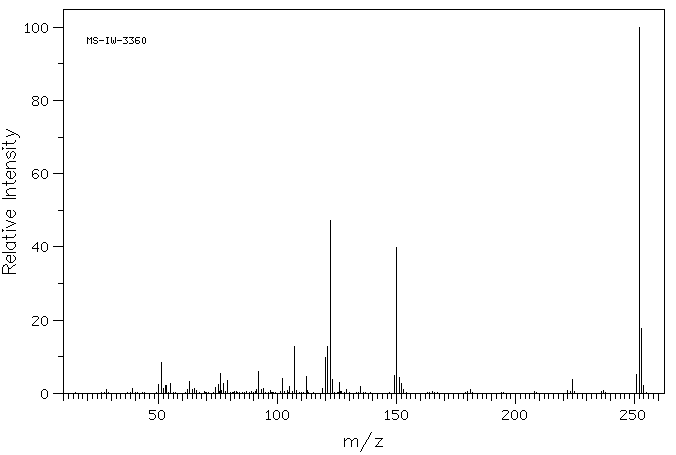
质谱MS
-
碳谱13CNMR
-
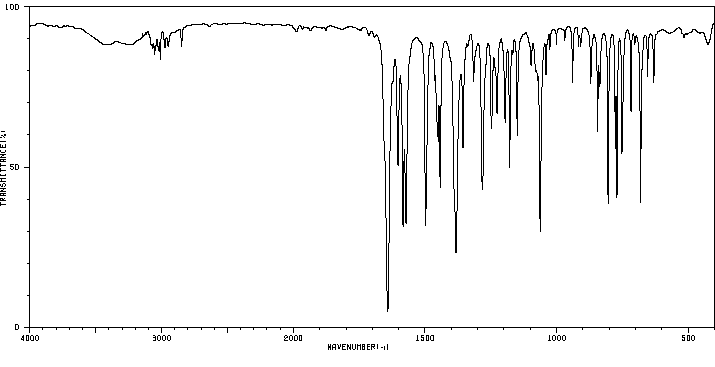
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷