十四烷二酸二甲酯 | 19812-63-6
中文名称
十四烷二酸二甲酯
中文别名
十四烷二酸二乙酯
英文名称
diethyl tetradecanedioate
英文别名
tetradecanedioic acid diethyl ester;diethyl 1,12-dodecanedicarboxylate;Tetradecandisaeure-diaethylester
CAS
19812-63-6
化学式
C18H34O4
mdl
MFCD00009205
分子量
314.466
InChiKey
XXYWYFSQJPFXBD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:29-31 °C(lit.)
-
沸点:374.13°C (rough estimate)
-
密度:0.9862 (rough estimate)
-
闪点:223 °F
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):5.7
-
重原子数:22
-
可旋转键数:17
-
环数:0.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S24/25
-
WGK Germany:3
SDS
1.1 产品标识符
: Diethyl tetradECanedioate
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C18H34O4
分子式
: 314.46 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 29 - 31 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
106.00 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
酸, 碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: Diethyl tetradECanedioate
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C18H34O4
分子式
: 314.46 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 29 - 31 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
106.00 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
酸, 碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 辛二酸氢乙酯 Ethyl hydrogen suberate 14113-01-0 C10H18O4 202.251 —— azelaic acid monoethyl ester 1593-55-1 C11H20O4 216.277 十四烷二酸 1,14-tetradecanedioic acid 821-38-5 C14H26O4 258.358 辛二酸 Suberic acid 505-48-6 C8H14O4 174.197 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,12-dodecane-dicarboxylic acid monoethylester 92264-70-5 C16H30O4 286.412 —— ethyl 14-hydroxytetradecanoate 910631-01-5 C16H32O3 272.428 —— 14-chloroketo-tetradecacarboxylic acid ethylester 14812-21-6 C16H29ClO3 304.857 14-羟基豆蔻酸 14-hydroxymyristic acid 17278-74-9 C14H28O3 244.375
反应信息
-
作为反应物:参考文献:名称:基于非对映选择性共轭加成的(R)-(–)-muscone的不对称合成摘要:(R)-(-)-Muscone是通过立体选择性的方式合成的,方法是将非对映体选择性共轭加成至(R,R)-环己烷-1,2-二醇的环状α,β-不饱和酯,并伴随自发Dieckmann缩合。DOI:10.1039/c39910001438
-
作为产物:参考文献:名称:Iwakura et al., Nippon Kagaku Zasshi, 1957, vol. 78, p. 1504摘要:DOI:
文献信息
-
Influence of Positional Isomers on the Macroscale and Nanoscale Architectures of Aggregates of Racemic Hydroxyoctadecanoic Acids in Their Molecular Gel, Dispersion, and Solid States作者:Shibu Abraham、Yaqi Lan、Ricky S. H. Lam、Douglas A. S. Grahame、Jennifer Jae Hee Kim、Richard G. Weiss、Michael A. RogersDOI:10.1021/la204412t日期:2012.3.20Inter/intramolecular hydrogen bonding of a series of hydroxystearic acids (HSAs) are investigated. Self-assembly of molecular gels obtained from these fatty acids with isomeric hydroxyl groups is influenced by the position of the secondary hydroxyl group. 2-Hydroxystearic acid (2HSA) does not form a molecular dimer, as indicated by FT-IR, and growth along the secondary axis is inhibited because the secondary hydroxyl
-
METHOD OF PRODUCING SATURATED ALKYL ESTERS/ACIDS申请人:West Ryan Michael公开号:US20130066089A1公开(公告)日:2013-03-14Disclosed herein is the production of saturated alkyl esters or acids from furan materials. The starting compounds contain furan, ketone, and ester or acid functional groups and may be biologically-derived. The method includes hydrogenating the starting compound to form a reduced mixture. The method further includes hydrodeoxygenation of the reduced mixture to yield a saturated alkyl ester or acid. The saturated alkyl ester or acid can be unbranched or branched. The ester and acid products have a wide variety of applications and may be further processed into surfactants, solvents, and lubricants suitable for use in consumer products.
-
Synthesis of 2,4-Furanophanes by Palladium-Catalyzed Macrocyclization Reactions of 1,n-Diallenyl Diketones作者:A. Stephen K. Hashmi、Lothar Schwarz、Michael BolteDOI:10.1002/ejoc.200300792日期:2004.5The 1,n-diallenyl diketones 8a−j were prepared and subjected to reactions with the [PdCl2(MeCN)2] catalyst. Upon decreasing the length of the bridge between the allenyl ketone units, first we obtained the (E) isomer 10 as the macrocyclic product and then the (Z) isomer 11 accompanied by the exocyclic double bond isomer 12. In all cases, the open-chained 1,n-difuryl alkanes were isolated as side-products制备 1,n-二烯基二酮 8a-j 并使其与 [PdCl2(MeCN)2] 催化剂反应。在减少丙二烯基酮单元之间的桥的长度后,首先我们得到 (E) 异构体 10 作为大环产物,然后得到 (Z) 异构体 11 和环外双键异构体 12。在所有情况下,开环-链状 1,n-二呋喃烷烃作为副产物被分离出来。具有更长桥连和醚官能团的二烯基二酮 19 和 24 的类似制备和转化产生了 20 至 52 元大环 21a、21b、26a 和 26b,仅具有预期的 (E) 构型双键。呋喃衍生物的闭环,在其 2-和 4-位的取代基中具有乙烯基,通过 Ru 催化的烯烃复分解反应生成相关产物,其产率类似于 Pd 催化的大环化,但提供了两种双键异构体的混合物。(© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2004)
-
Cytotoxic Glycosylated Fatty Acid Amides from a <i>Stelletta</i> sp. Marine Sponge作者:Victoria Peddie、Kentaro Takada、Shujiro Okuda、Yuji Ise、Yasuhiro Morii、Nobuhiro Yamawaki、Tomohiro Takatani、Osamu Arakawa、Shigeru Okada、Shigeki MatsunagaDOI:10.1021/acs.jnatprod.5b00795日期:2015.11.25We have discovered new glycosylated fatty acid amides, stellettosides, from a Stelletta sp. marine sponge. They were detected through LC-MS analysis of the extract combined with the cytotoxicity assay of the prefractionated sample. Their planar structures were determined by analyses of the NMR and tandem FABMS data. Stellettosides A1 and A2 (1 and 2) as well as stellettosides B1–B4 (3–6) were obtained我们已经发现了新的糖基化的脂肪酸酰胺,stellettosides,从Stelletta SP。海洋海绵。通过提取物的LC-MS分析和预分离样品的细胞毒性测定相结合,对它们进行了检测。通过分析NMR和串联FABMS数据确定了它们的平面结构。硬脂甙A1和A2(1和2)以及硬脂甙B1-B4(3 – 6)是不可分离的混合物。仔细分析每种混合物的NMR和串联FABMS数据,以及将串联FABMS数据与合成模型化合物的数据进行比较,使我们能够确定混合物中各成分的结构。手性衍生化后,通过LC-MS确定单糖单元的绝对构型。通过异亚丙基衍生物的1 H NMR数据确定脂肪酸链上邻位氧化次甲基的相对构型。(stellettosides B1-B4的混合物3 - 6)显示出适度的细胞毒性活性对HeLa细胞的IC 50 浓度为9μM,而硬脂甙A1和A2(1和2)的混合物在10μM的浓度下没有活性。
-
Synthesis, Lipophilicity Studies and Antibacterial Properties of Some Novel Quaternary Ammonium Salts.作者:Theodora SIATRA-PAPASTAIKOUDI、Aspasia PAPADAKI-VALIRAKI、Anna TSANTILI-KAKOULIDOU、Leonidas TZOUVELEKIS、Andreas MENTISDOI:10.1248/cpb.42.392日期:——The synthesis of some novel quaternary ammonium salts, derivatives of 15-phenyl-decapentanoic acid, is described. Their lipophilicity was estimated applying the Hansch-Leo fragmental procedure and measured by means of reversed phase thin layer chromatography. All compounds were tested for their antibacterial activity against Gram positive and gram negative microorganisms. The less lipophilic compounds
表征谱图
-
氢谱1HNMR
-
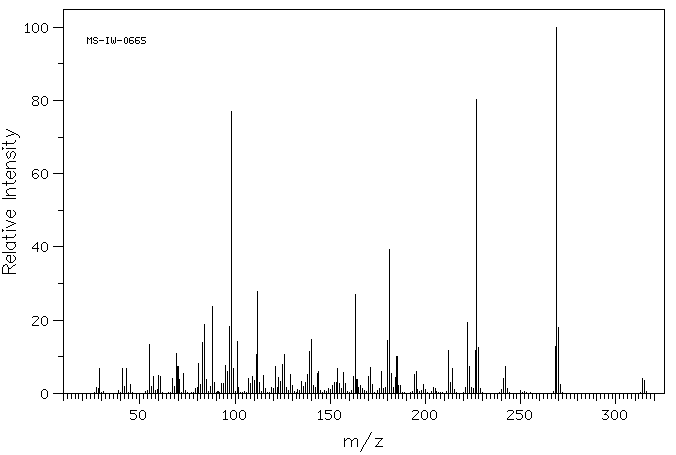
质谱MS
-
碳谱13CNMR
-
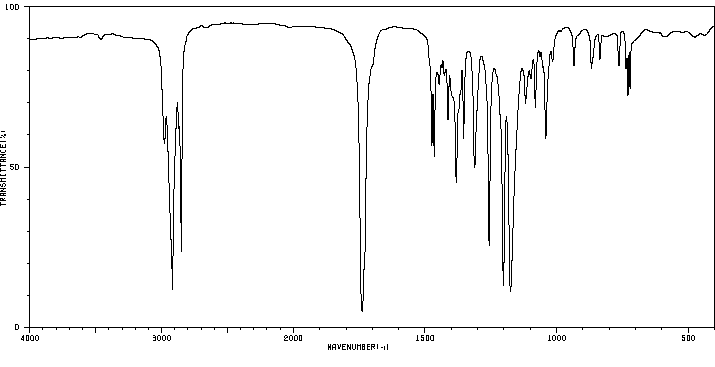
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯