6-甲基-6-庚烯-4-炔-3-醇 | 95764-76-4
中文名称
6-甲基-6-庚烯-4-炔-3-醇
中文别名
——
英文名称
6-methyl-6-hepten-4-yn-3-ol
英文别名
6-methylhept-6-en-4-yn-3-ol
CAS
95764-76-4
化学式
C8H12O
mdl
MFCD00041590
分子量
124.183
InChiKey
DUDZQARPXNGCOB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:87°C 14mm
-
保留指数:972
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
海关编码:2905290000
-
安全说明:S26,S36/37/39
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-(-)-6-methyl-6-hepten-4-yn-3-ol 135758-83-7 C8H12O 124.183
反应信息
-
作为反应物:参考文献:名称:通过乙烯基丙烯烯和磺酰基氰化物的[4 + 2]环加成反应合成高度取代的吡啶。摘要:描述了合成多取代吡啶的收敛策略。乙烯基烯与Diels-Alder环加成物中的市售芳基磺酰基氰化物结合,生成异吡啶环加成物,其在进一步加热或添加碱后转化为吡啶。2-磺酰吡啶产物与氧和碳亲核试剂进行亲核芳族取代反应,以提供进入各种高度取代的吡啶的途径。DOI:10.1021/acs.joc.9b02628
-
作为产物:描述:丙醛 、 9-(3-Methylbut-3-en-1-yn-1-yl)-9-borabicyclo[3.3.1]nonane 在 C.I.酸性橙108 作用下, 以 正戊烷 为溶剂, 反应 0.5h, 以81%的产率得到6-甲基-6-庚烯-4-炔-3-醇参考文献:名称:Organoboranes. 38. A facile and highly efficient addition of B-1-alkynyl-9-borabicyclo[3.3.1]nonanes to aldehydes and ketones: an exceptionally chemoselective synthesis of propargylic alcohols摘要:DOI:10.1021/jo00210a003
文献信息
-
Ene-yne unsaturated compounds as accelerators for hydrosilation申请人:DOW CORNING CORPORATION公开号:EP0751140A2公开(公告)日:1997-01-02A hydrosilation process where a silicon hydride is reacted with an unsaturated reactant in the presence of a platinum catalyst and an accelerator selected from ene-yne unsaturated compounds. The accelerators are especially useful for facilitating the hydrosilation of unsaturated reactants where the unsaturation is in the internal portion of the reactant's structure, for example, as in cyclopentene or cyclohexene.
-
Diastereoselective [4 + 1] Cycloaddition of Alkenyl Propargyl Acetates with CO Catalyzed by [RhCl(CO)<sub>2</sub>]<sub>2</sub>作者:Wei Chen、Jia-Hui Tay、Xiao-Qi Yu、Lin PuDOI:10.1021/jo3009403日期:2012.7.20A class of alkenyl propargyl acetates, RCH(OAc)C CC(CH3)=CH2 (5), are found to undergo [4 + 1] cycloaddition with CO (1 atm) in the presence of [RhCl(CO)(2)](2) in refluxing 1,2-dichloroethane to give cyclopentenones (6) in good yields. It has been demonstrated that, when the R group of 5 is a phenyl group bearing o-electron-withdrawing substituents up to 10:1 diastereoselectivity and 96% yield can be achieved for the [4 + 1] cycloaddition. This process provides a convenient method to construct highly functionalized cyclopentenones that are useful in organic synthesis.
-
Nonenzymatic Kinetic Resolution of Propargylic Alcohols by a Planar−Chiral DMAP Derivative: Crystallographic Characterization of the Acylated Catalyst作者:Beata Tao、J. Craig Ruble、Diego A. Hoic、Gregory C. FuDOI:10.1021/ja9906958日期:1999.6.1
-
Enantioselective esterifications of unsaturated alcohols mediated by a lipase prepared from Pseudomonas sp作者:Kevin Burgess、Lee D. JenningsDOI:10.1021/ja00016a032日期:1991.7Competition experiments and measurements of enantioselectivities were used to develop a simple active-site model (Figure 1) for resolutions of beta-hydroxy-alpha-methylene carbonyl compounds III via acyl transfers mediated by lipase from Pseudomonas sp. (AK). Further experiments were used to test and refine this model with respect to resolutions of allylic, propargylic, homopropargylic, and other alcohols (Tables I-IV, respectively). The model proved extremely reliable for predicting the sense of the asymmetric induction, and the combined data collected in this paper give an indication of what structural features of the substrates can be correlated with high enantioselectivities in these resolutions. Furthermore, the results account for the conspicuous reversal of enantioselectivity previously observed in resolutions of gamma-hydroxy-alpha,beta-unsaturated esters 35. Kinetic resolutions of two substrates (allenol 14 and dienol 9) via asymmetric epoxidations were performed for comparison with the methodology presented in this paper.
-
BROWN, H. C.;MOLANDER, G. A.;SINGH, SHANKAR, M.;RACHERLA, U. S., J. ORG. CHEM., 1985, 50, N 10, 1577-1582作者:BROWN, H. C.、MOLANDER, G. A.、SINGH, SHANKAR, M.、RACHERLA, U. S.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
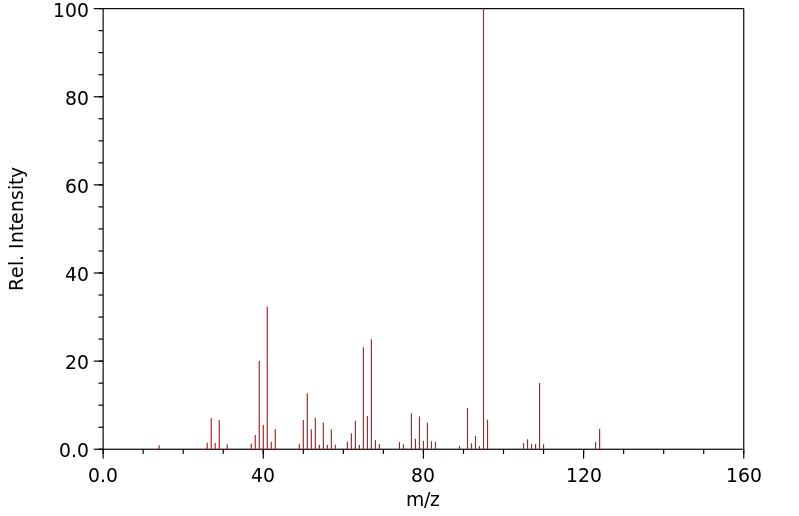
质谱MS
-
碳谱13CNMR
-
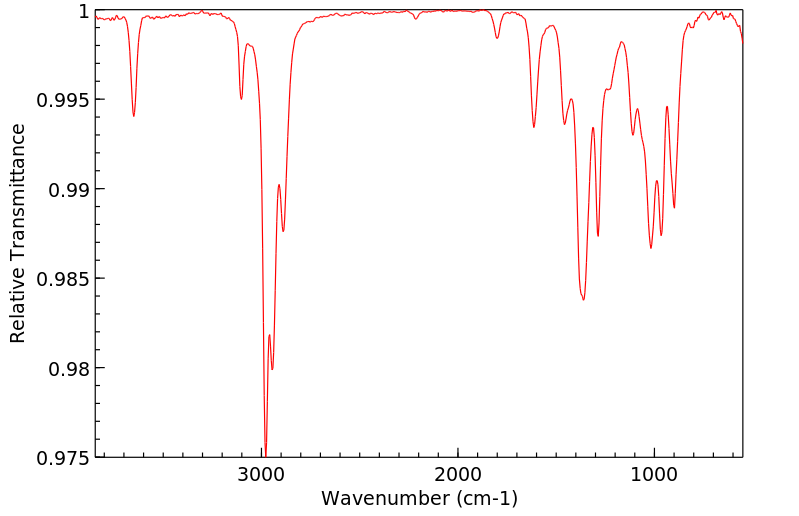
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷