3',4'-dibenzyloxy-2'-methoxyacetophenone | 27829-92-1
中文名称
——
中文别名
——
英文名称
3',4'-dibenzyloxy-2'-methoxyacetophenone
英文别名
2-methyl-3,4-dibenzyloxyacetophenone;1-[2-Methoxy-3,4-bis(phenylmethoxy)phenyl]ethanone
CAS
27829-92-1
化学式
C23H22O4
mdl
——
分子量
362.425
InChiKey
JHEVSXTVVGAQIJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:514.9±45.0 °C(Predicted)
-
密度:1.152±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:27
-
可旋转键数:8
-
环数:3.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3,4-dibenzyloxy-2-hydroxyacetophenone 2652-27-9 C22H20O4 348.398 2',3',4'-三羟基苯乙酮 2',3',4'-trihydroxyacetophenone 528-21-2 C8H8O4 168.149 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,4-二羟基-2-甲氧基苯乙酮 3',4'-dihydroxy-2'-methoxyacetophenone 27829-93-2 C9H10O4 182.176
反应信息
-
作为反应物:描述:3',4'-dibenzyloxy-2'-methoxyacetophenone 在 palladium on activated charcoal 氢气 作用下, 以83%的产率得到3,4-二羟基-2-甲氧基苯乙酮参考文献:名称:Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents摘要:The DPPH (2,2-diphenyl-l-picrylhydrazyl) radical scavenging activity of protocatechuic acid (3,4-dihydroxybenzoic acid) and its related catechols was examined. Compounds possessing strong electron-withdrawing substituents showed high activity. NMR analysis of the reaction mixtures of catechols and DPPH radical in methanol showed the formation of methanol adducts. The results suggest that high radical scavenging activity of catechols in alcohol is due to a nucleophilic addition of an alcohol molecule on o-quinones, which leads to a regeneration of a catechol structure. Furthermore, the radical scavenging activity in alcohols would largely depend on the electron-withdrawing/donating substituents, Since they affect the susceptibility toward nucleophilic attacks on o-quinone. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2005.06.040
-
作为产物:描述:2',3',4'-三羟基苯乙酮 在 potassium carbonate 、 N,N-二异丙基乙胺 作用下, 以 甲醇 、 正己烷 、 丙酮 、 乙腈 为溶剂, 反应 18.0h, 生成 3',4'-dibenzyloxy-2'-methoxyacetophenone参考文献:名称:Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents摘要:The DPPH (2,2-diphenyl-l-picrylhydrazyl) radical scavenging activity of protocatechuic acid (3,4-dihydroxybenzoic acid) and its related catechols was examined. Compounds possessing strong electron-withdrawing substituents showed high activity. NMR analysis of the reaction mixtures of catechols and DPPH radical in methanol showed the formation of methanol adducts. The results suggest that high radical scavenging activity of catechols in alcohol is due to a nucleophilic addition of an alcohol molecule on o-quinones, which leads to a regeneration of a catechol structure. Furthermore, the radical scavenging activity in alcohols would largely depend on the electron-withdrawing/donating substituents, Since they affect the susceptibility toward nucleophilic attacks on o-quinone. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2005.06.040
文献信息
-
Structural confirmation of spiroelliptin from Iryanthera elliptica by synthesis作者:Anselmo A. Morais、Raimundo Braz Fo、Otto R. GottliebDOI:10.1016/0031-9422(85)80049-2日期:1985.11Abstract Spiroelliptin, a spiro[cyclohexadienone-1,1′-tetralin] from Iryanthera elliptica , was synthesized by a novel process which involved the catalytic hydrogenation of the appropriate chalcone. This and other spiro[methoxycyclobexadienone-1,1′-tetralin] derivatives, obtained by the same process or by the oxidative coupling of the appropriate 1,3-diarylpropanes, were used as models in the compilation
表征谱图
-
氢谱1HNMR
-
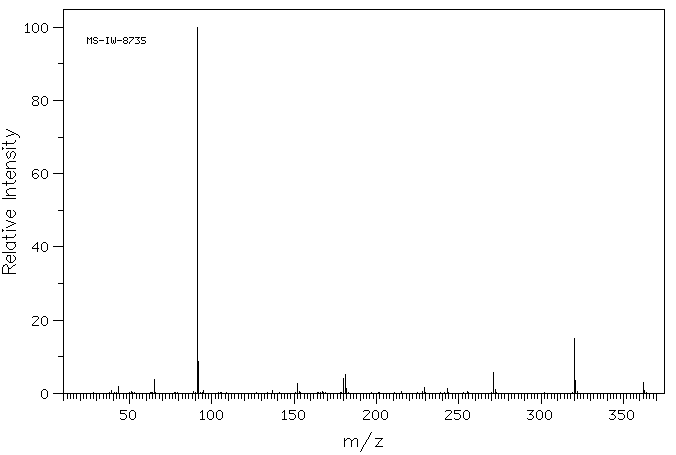
质谱MS
-
碳谱13CNMR
-
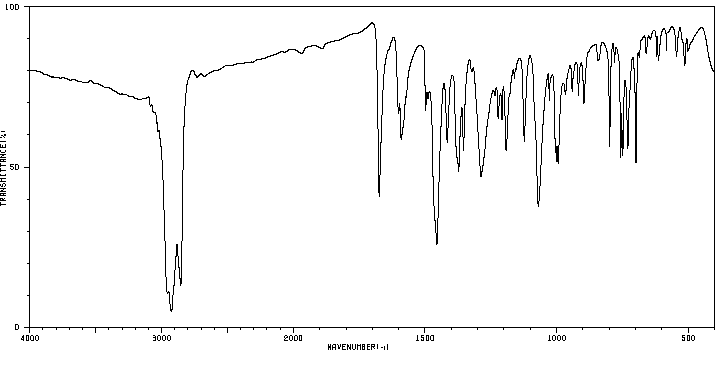
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷