1,2,3,4-环戊四羧酸二酐 | 6053-68-5
中文名称
1,2,3,4-环戊四羧酸二酐
中文别名
1,2,3,4-环戊烷四甲酸二酐;1,2,3,4-环戊烷四羧酸二酐
英文名称
1,2,3,4-cyclopentanetetracarboxylic acid dianhydride
英文别名
1,2,3,4-cyclopentanetetracarboxylic dianhydride;1,2,3,4-cyclopentane tetracarboxylic acid dianhydride;1H-Cyclopenta[1,2-c:3,4-c']difuran-1,3,4,6(3aH)-tetrone, tetrahydro-;4,10-dioxatricyclo[6.3.0.02,6]undecane-3,5,9,11-tetrone
CAS
6053-68-5
化学式
C9H6O6
mdl
MFCD00046872
分子量
210.143
InChiKey
NLWBEORDOPDUPM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:213 °C
-
沸点:527℃
-
密度:1.677
-
闪点:244℃
-
溶解度:溶于丙酮
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:86.7
-
氢给体数:0
-
氢受体数:6
安全信息
-
危险品标志:Xi
-
安全说明:S25
-
危险类别码:R36/37
-
海关编码:2932190090
SDS
Section I.Chemical Product and Company Identification
Chemical Name 1,2,3,4-Cyclopentanetetracarboxylic Dianhydride
Portland OR
Synonym 1H-Cyclopenta[1,2-c:3,4-c']difuran-1,3,4,6(3aH)-tetrone,
tetrahydro- (CA INDEX)
C9H6O6
Chemical Formula
CAS Number 6053-68-5
Section II. Composition and Information on Ingredients
Toxicology Data
Chemical Name CAS Number Percent (%) TLV/PEL
Min. 98.0 (T) Not available. Not available.
1,2,3,4-Cyclopentanetetracarboxylic Dianhydride 6053-68-5
Section III. Hazards Identification
Acute Health Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling
this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
Inhalation
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Ingestion
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
May be combustible at high temperature. Auto-Ignition
Flammability
Flash Points Flammable Limits Not available.
Not available.
These products are toxic carbon oxides (CO, CO2).
Combustion Products
Fire Hazards Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
1,2,3,4-Cyclopentanetetracarboxylic Dianhydride
Section VI. Accidental Release Measures
Spill Cleanup Irritating material.
Use a shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
Instructions
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
IRRITANT. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in a
Handling and Storage
dry, cool place. Avoid excessive heat and light. Do not breathe dust.
Information
Always store away from incompatible compounds such as oxidizing agents, moisture.
Section VIII. Exposure Controls/Personal Protection
Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended
Engineering Controls
exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants
below the exposure limit.
Splash goggles. Lab coat. Dust respirator. Gloves. Suggested protective clothing might not be sufficient; consult a specialist
Personal Protection
BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solid. (White crystal~crystalline powder) Solubility
Physical state @ 20°C Soluble in: Acetone.
Not available.
Specific Gravity
Molecular Weight 210.14 Partition Coefficient
Not available.
Boiling Point Not available. Vapor Pressure Not applicable.
226°C (438.8°F) Not available.
Melting Point Vapor Density
Refractive Index Not available. Volatility Not available.
Not available. Not available.
Critical Temperature Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
This material is stable if stored under proper conditions. (See Section VII for instructions)
Stability
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents, moisture.
Section XI. Toxicological Information
Not available.
RTECS Number
Eye Contact. Ingestion. Inhalation.
Routes of Exposure
Not available.
Toxicity Data
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye
Acute Toxic Effects
is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling
this compound.
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Continued on Next Page
1,2,3,4-Cyclopentanetetracarboxylic Dianhydride
Section XIII. Disposal Considerations
Waste Disposal Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
Not a DOT controlled material (United States).
DOT Classification
PIN Number Not applicable.
Proper Shipping Name Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list.
(EPA)
WHMIS Classification On NDSL.
(Canada)
EINECS Number (EEC) 227-964-7
EEC Risk Statements R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,4-环戊烷四羧酸 cyclopentane-1,2,3,4-tetracarboxylic acid 3724-52-5 C9H10O8 246.174 纳迪克酸酐 5-norbornene-2,3-dicarboxylic anhydride 826-62-0 C9H8O3 164.161
反应信息
-
作为反应物:参考文献:名称:苯马来酰亚胺光加合物米丁domide的同类和前药的合成作为潜在的抗肿瘤剂。2。摘要:通过几种不同的方法合成了高度不溶的二酰亚胺抗肿瘤剂米坦度胺(1b)的潜在前药。米线度胺和甲醛之间的缩合反应可干净地得到稳定的双(羟甲基)化合物7a,该化合物部分溶于水(约0.8%),并在P-388筛查中显示出改进的活性。当该化合物用仲胺处理时,可以分离出高收率的曼尼希碱。来自N-甲基哌嗪的化合物(7b)具有优异的性能,可用于临床试验。与其他醛的缩合要么没有反应,要么没有活性差的化合物。由7a和琥珀酸酐制备水溶性酯,但是效力和活性降低。1a的双键与臭氧的氧化产生了惰性二酸,而二氢化合物的活性与烯烃相同。当用马来酰亚胺光解其它芳族化合物(苯甲醚,对二甲苯,均三甲苯)时,发现所得的光产物是无活性的。由相应的酸酐合成了来自其他环系统的二酰亚胺,发现它们是无活性的。然而,这些(12a)之一的双(羟甲基)衍生物在P-388筛选中是有活性的。DOI:10.1021/jm00161a006
-
作为产物:描述:参考文献:名称:一种1,2,3,4-环戊烷四羧酸二酐的制备方法摘要:本发明公开了一种1,2,3,4‑环戊烷四羧酸二酐的制备方法,本发明属于精细化学品制备方法领域。其主要技术方案是以顺丁烯二酸酐和环戊二烯为起始原料,低温下经1,4‑加成反应合成中间产物5‑降冰片烯‑2,3‑二羧酸酐;再通过臭氧氧化、水解、双氧水氧化反应得到环戊烷四羧酸;最后以乙酸酐做脱水剂,脱水关环合成目标产物1,2,3,4‑环戊烷四羧酸二酐。本方案相对于传统工艺,具有反应条件温和、产品纯度高、收率高、无污染等优点,适合大规模工业化生产。公开号:CN109627252A
文献信息
-
[EN] POLYMERS FOR USE IN ELECTRONIC DEVICES<br/>[FR] POLYMÈRES DESTINÉS À ÊTRE UTILISÉS DANS DES DISPOSITIFS ÉLECTRONIQUES申请人:DU PONT公开号:WO2019222304A1公开(公告)日:2019-11-21There is disclosed an acid dianhydride having Formula (IV). In Formula IV: Rd represents a tetracarboxylic acid component residue; Re represents a diamine residue; and m is an integer from 1-20.
-
Inkjet ink申请人:Satou Hiroyuki公开号:US20080085361A1公开(公告)日:2008-04-10The invention relates to a method for manufacturing an inkjet ink including a polyamic acid (A), including a step of at least reacting one or more compounds selected from the group of a monoamine (a3) and a compound having one acid anhydride group (a4) with a compound having two or more acid anhydride groups (a1) and a diamine (a2).该发明涉及一种制造喷墨墨水的方法,包括至少将来自单胺(a3)和具有一个酸酐基团(a4)的化合物组合的群体中选择的一个或多个化合物与具有两个或更多酸酐基团(a1)和二胺(a2)反应的聚酰胺酸(A)。
-
Polyfunctional epoxy compound申请人:Endo Yuki公开号:US09428606B2公开(公告)日:2016-08-30There is provided an epoxy resin composition having low viscosity and a high cationic curing property. An epoxy compound of Formula (1): (in Formula (1), A is an (n4)-valent C4-20 linear hydrocarbon group optionally containing an epoxy group, an (n4)-valent C4-20 cyclic hydrocarbon group optionally containing an epoxy group, or an (n4)-valent group of a combination of the linear hydrocarbon group and the cyclic hydrocarbon group; R1 and R2 are each independently a hydrogen atom or a C1-10 alkyl group; n1 is an integer of 2 to 6; n2 is an integer of 2; n3 is an integer of 1; and n4 is an integer of 3 to 8). For example, A is an (n4)-valent organic group formed by removing (n4) hydrogen atoms from butane, pentane, or hexane, or an (n4)-valent organic group formed by removing (n4) hydrogen atoms from cyclobutane, cyclopentane, cyclohexane, epoxycyclohexane, alkyl-substituted epoxycyclohexane, bicycloheptene, or bicyclooctene.
-
Liquid crystal aligning agent for photoalignment, liquid crystal alignment film and liquid crystal display device using it, and diamine and polymer申请人:JNC CORPORATION公开号:US10968392B2公开(公告)日:2021-04-06Provided are a liquid crystal alignment film capable of being given anisotropy through photoalignment and stable to light, and a liquid crystal display device capable of keeping a high voltage holding ratio without degradation of display quality even when exposed to strong light for a long period of time. To provide these, used is a liquid crystal aligning agent for photoalignment which contains a polymer having a structural unit containing a photoreactive structure and in which the structural unit containing a photoreactive structure undergoes chemical reaction by heating.提供了一种液晶取向膜,能够通过光取向获得各向异性,并且对光稳定,并且提供了一种液晶显示装置,即使长时间暴露在强光下也能保持高电压保持比率而不会降低显示质量。为了实现这些,使用了一种用于光取向的液晶取向剂,其中包含具有光反应结构的结构单元的聚合物,并且具有光反应结构的结构单元通过加热发生化学反应。
-
CAGE-SHAPED CYCLOPENTANOIC DIANHYDRIDE, METHOD FOR PRODUCTION THEREOF, AND POLYIMIDE申请人:NISSAN CHEMICAL INDUSTRIES, LTD.公开号:US20140114027A1公开(公告)日:2014-04-24A cage 1,2,3,4-cyclopentanetetracarboxylic acid (1,3:2,4)-dianhydride compound represented by formula [1], and a polyimide obtained by condensing the compound with a diamine. With the compound, it is possible to provide a polyimide which shows no absorption in the ultraviolet region and is highly transparent to light, has high insulating properties, has improved heat resistance and processability, and has excellent solubility in organic solvents. (In formula [1], R 1 and R 2 each independently represents a hydrogen atom, a halogen atom, or a C 1-10 alkyl.)化学式为[1]的1,2,3,4-环戊烷四羧酸(1,3:2,4)-二酐化合物和通过该化合物与二胺缩合制得的聚酰亚胺。使用该化合物,可以提供一种聚酰亚胺,该聚酰亚胺在紫外线区域不吸收,并且对光高度透明,具有高绝缘性能,改善了耐热性和加工性,并且在有机溶剂中溶解性优异。(在公式[1]中,R1和R2各自独立地表示氢原子、卤素原子或C1-10烷基。)
表征谱图
-
氢谱1HNMR
-
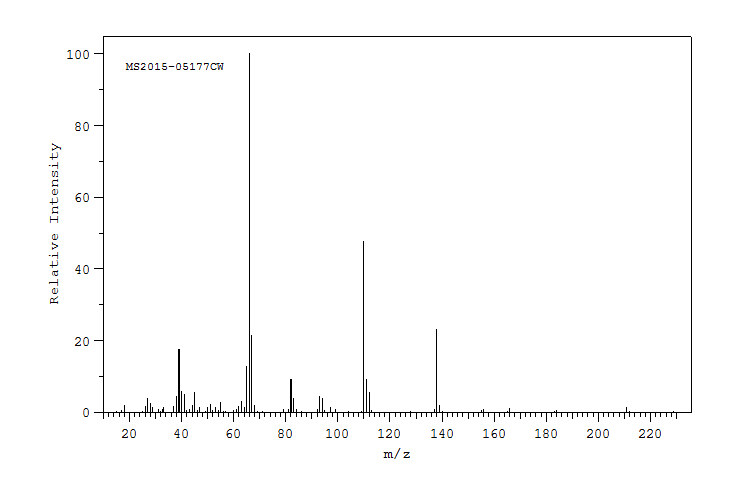
质谱MS
-
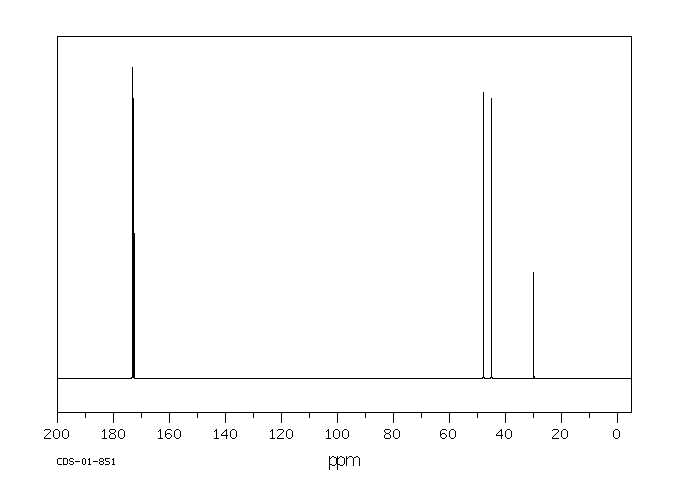
碳谱13CNMR
-
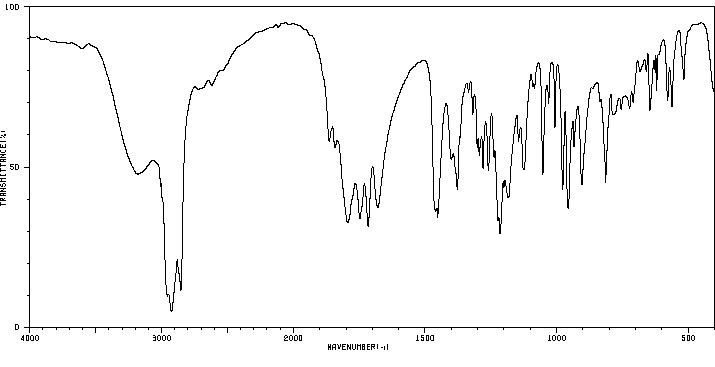
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸