亚乙基环丁烷 | 1528-21-8
中文名称
亚乙基环丁烷
中文别名
——
英文名称
ethylidenecyclobutane
英文别名
Ethylidencyclobutan;ethylenecyclobutane;ethylidene-cyclobutane;Aethyliden-cyclobutan;1-Aethyliden-cyclobuten;Aethylidencyclobutan
CAS
1528-21-8
化学式
C6H10
mdl
——
分子量
82.1454
InChiKey
FYHISAJRLZLAQP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:80.26 °C
-
密度:0.7678 g/cm3(Temp: 204 °C)
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Finkel'shtein,E.Sh. et al., Doklady Chemistry, 1977, vol. 232, p. 98 - 101摘要:DOI:
-
作为产物:参考文献:名称:Finkel'shtein,E.Sh. et al., Doklady Chemistry, 1977, vol. 232, p. 98 - 101摘要:DOI:
文献信息
-
Alcohol Synthesis by Cobalt-Catalyzed Visible-Light-Driven Reductive Hydroformylation作者:Connor S. MacNeil、Lauren N. Mendelsohn、Tyler P. Pabst、Gabriele Hierlmeier、Paul J. ChirikDOI:10.1021/jacs.2c07745日期:2022.10.26A cobalt-catalyzed reductive hydroformylation of terminal and 1,1-disubstituted alkenes is described. One-carbon homologated alcohols were synthesized directly from CO and H2, affording anti-Markovnikov products (34–87% yield) with exclusive regiocontrol (linear/branch >99:1) for minimally functionalized alkenes. Irradiation of the air-stable cobalt hydride, (dcype)Co(CO)2H (dcype = dicyclohexylphosphinoethane)
-
Derfer; Greenlee; Boord, Journal of the American Chemical Society, 1949, vol. 71, p. 180作者:Derfer、Greenlee、BoordDOI:——日期:——
-
Vogel; Mueller, Justus Liebigs Annalen der Chemie, 1958, vol. 615, p. 29,33作者:Vogel、MuellerDOI:——日期:——
-
Reactions of Ruthenium Carbenes of the Type (PPh3)2(X)2Ru:CH-CH:CPh2 (X = Cl and CF3COO) with Strained Acyclic Olefins and Functionalized Olefins作者:Zhe Wu、SonBinh T. Nguyen、Robert H. Grubbs、Joseph W. ZillerDOI:10.1021/ja00125a010日期:1995.5Ruthenium carbene complexes of the type (PPh(3))(2)(X)(2)Ru=CH-CH=CPh(2) (1, X = Cl; 2, X = CF3COO) can react with strained acyclic olefins and functionalized olefins. Complex 1 reacts with methylenecyclopropane and methylenecyclobutane and their derivatives to generate new active ring-opening metathesis polymerization (ROMP) catalysts. The product from the reaction between 1 and ethyl vinyl ether decomposes through a bimolecular coupling pathway, Complex 2 reacts with functionalized terminal olefins, such as alkyl vinyl ether, enamine, and alkyl vinyl sulfide to give hetero-substituted carbene complexes. However, in the case of alkyl vinyl ether, the resulting alkoxymethylenecarbene complex decomposes to ruthenium carbonyl species at room temperature. Complex 2 can also isomerize allylic vinyl ether or alcohol. Aromatic amines can react with 2 by first coordinating trans to the carbene ligand; a fact which indicates that the potential coordination site for olefin metathesis may be trans to the carbene moiety. The reactivity pattern of 2 with functionalized vinyl olefins suggests that this reaction is best understood under the context of a Lewis acid/Lewis base interaction.
-
Siegel, Herbert; Eisenhuth, Ludwig; Hopf, Henning, Chemische Berichte, 1985, vol. 118, p. 597 - 612作者:Siegel, Herbert、Eisenhuth, Ludwig、Hopf, HenningDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
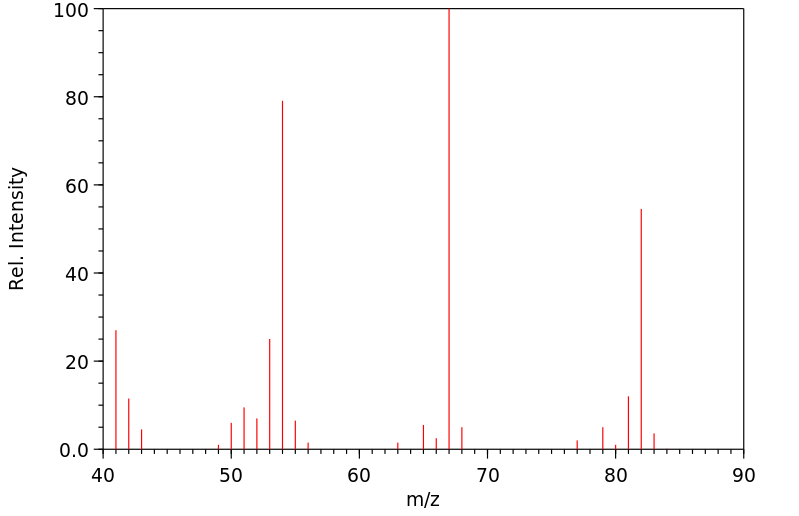
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-