4-丙基-1,6-庚二烯-4-醇 | 52939-61-4
中文名称
4-丙基-1,6-庚二烯-4-醇
中文别名
——
英文名称
4-hydroxy-4-n-propyl-1,6-heptadiene
英文别名
4-n-propyl-1,6-heptadien-4-ol;4-propyl-hepta-1,6-dien-4-ol;Diallyl-propyl-carbinol;4-Propyl-1,6-heptadien-4-ol;4-propylhepta-1,6-dien-4-ol
CAS
52939-61-4
化学式
C10H18O
mdl
MFCD00048189
分子量
154.252
InChiKey
UPDFVVLBKBYBOG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:192-194 °C
-
密度:0.879 g/cm3(Temp: 0 °C)
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:11
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
SDS
反应信息
-
作为反应物:描述:4-丙基-1,6-庚二烯-4-醇 在 potassium permanganate 作用下, 生成 4-propyl-heptane-1,2,4,6,7-pentaol参考文献:名称:Marko, Chemisches Zentralblatt, 1901, vol. 72, # I, p. 997摘要:DOI:
-
作为产物:参考文献:名称:Metal zinc-promoted gem -bisallylation of acid chlorides with allyl chlorides in the presence of chlorotrimethylsilane摘要:Treatment of acid chlorides (2) with allyl chlorides (1) in the presence of zinc dust and a catalytic amount of chlorotrimethylsilane (TMSCl) in THF brought about highly facile and effective coupling to give the corresponding gem-bisallylation products. 4-hydroxy-penta-1,6-dienes (3), in good to excellent yields. These reactions are assumed to proceed through allylzinc intermediates generated in situ. (C) 2002 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(02)01437-5
文献信息
-
γ-Substituted Secondary Organoalkaline Compounds and their Chlorinated Precursors: Synthetic Applications作者:José Barluenga、Josefa Flórez、Miguel YusDOI:10.1055/s-1985-31362日期:——The prepartion of γ-functionalised secondary organolkaline metal compounds starting from methyl 3-chlorobutanoate (obtained by addition of hydrogen chloride to commercially available methyl trans-2-butenoate) is described. Reactions of these metallated compounds with suitable electrophilic reagents leads to a variety of tertiary alcohol derivatives.
-
ALLYLATION OF ESTERS PROMOTED BY METALLIC DYSPROSIUM IN THE PRESENCE OF MERCURIC CHLORIDE作者:Yu Jia、Mingfu Zhang、Fenggang Tao、Jingyao ZhouDOI:10.1081/scc-120006467日期:2002.1ABSTRACT In the presence of mercuric chloride, the reactions of esters with allyl bromide and metallic dysprosium in anhydrous THF give diallyl alkyl carbinols in good yields. When γ-butyrolactone is used as the substrate, the corresponding product is 4-allyl-6-heptene-1, 4-diol.
-
Hypocholesterolemic agents VII: Inhibition of β-hydroxy-β-methylglutaryl-CoA reductase by monoesters of substituted glutaric acids作者:Marvin R. Boots、Yeong-Maw Yeh、Sharon G. BootsDOI:10.1002/jps.2600690507日期:1980.5A series of 1-(4-biphenylyl)pentyl hydrogen 3-alkylglutarates and 3-hydroxy-3-alkylglutarates was synthesized and assayed for inhibition of rat liver beta-hydroxy-beta-methylglutaryl-CoA reductase. Limited solubility of the monoesters in the enzyme assay system prevented the determination of the I50 values. However, the limited data indicated no significant changes in the activity of the analogs when
-
3-Alkyl-3-hydroxyglutaric Acids: A New Class of Hypocholesterolemic HMG CoA Reductase Inhibitors. 1作者:John S. Baran、Ivar Laos、Donna D. Langford、James E. Miller、Charlene Jett、Beatrice Taite、Elaine RohrbacherDOI:10.1021/jm50001a011日期:1985.5Derivatives of 3-hydroxy-3-methylglutaric acid (HMG), a portion of the substrate for HMG CoA reductase, were prepared and tested for their inhibitory action against rat liver HMG CoA reductase and for their hypocholesterolemic activity. Structure-dependent competitive inhibition was observed. Optimal structures had a free dicarboxylic acid with an alkyl group of 13-16 carbons at position 3. 3-n-Pentadecyl-3-hydroxyglutaric acid (3j) (IC50 = 50 microM) reduced serum cholesterol in the Triton-treated rat and HMG CoA reductase activity in the 20,25-diazacholesterol-treated rat.
-
Saizew,P.u.A., Justus Liebigs Annalen der Chemie, 1878, vol. 193, p. 362作者:Saizew,P.u.A.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
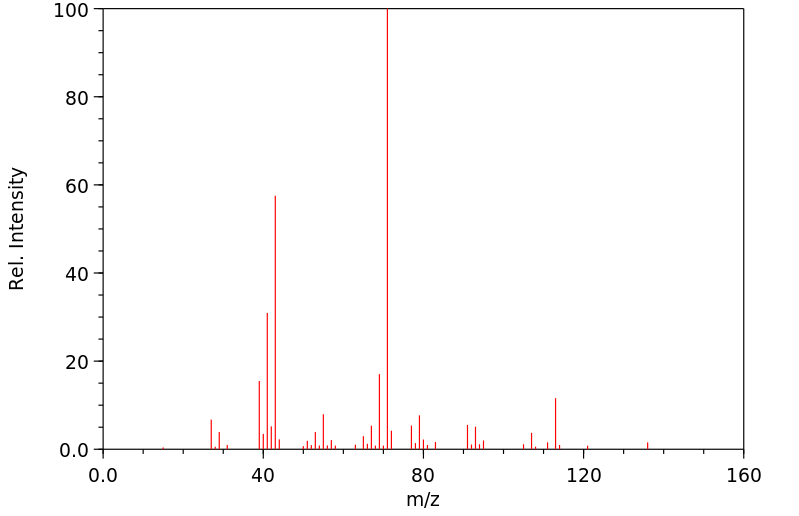
质谱MS
-
碳谱13CNMR
-
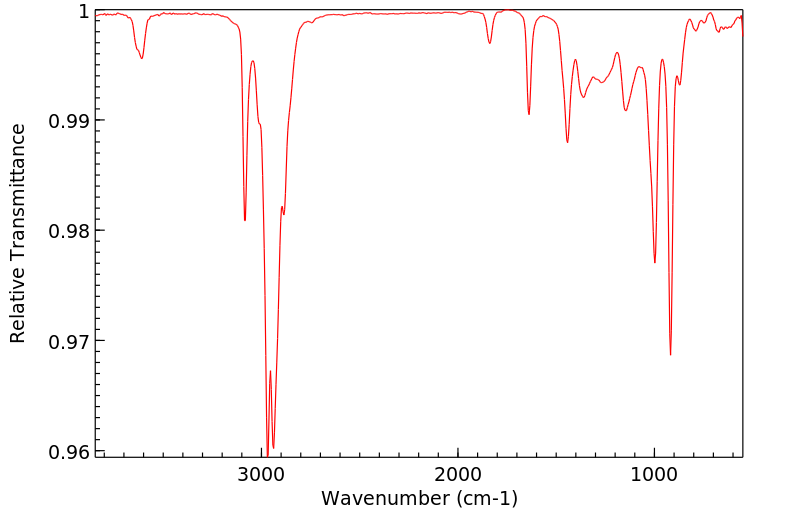
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷