4-羟基-4'-苄氧基二苯砜 | 63134-33-8
中文名称
4-羟基-4'-苄氧基二苯砜
中文别名
4-苄氧苯基-4'-羟基苯基砜;4-苯氧苯基-4'-羟基苯基砜;4-苄氧苯基-4’-羟基苯基砜;4-羟基-4"-苄氧基二苯砜;4-苄氧苯基-4"-羟基苯基砜
英文名称
4-benzyloxyphenyl-4-hydroxyphenyl sulfone
英文别名
4-((4-(benzyloxy)phenyl)sulfonyl)phenol;4-{[4-(benzyloxy)phenyl]sulfonyl}phenol;4-hydroxy-4'-benzyloxydiphenyl sulfone;4-benzyloxy-4'-hydroxydiphenylsulfone;4-(4-benzyloxyphenylsulfonyl)phenol;4-(4-phenylmethoxyphenyl)sulfonylphenol
CAS
63134-33-8
化学式
C19H16O4S
mdl
MFCD00185925
分子量
340.4
InChiKey
UWPJWCBDMZHMTN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:170 °C
-
沸点:558.6±40.0 °C(Predicted)
-
密度:1.304±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
-
稳定性/保质期:
在常温常压下稳定,应远离氧化剂。
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:24
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:72
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险品标志:Xn
-
WGK Germany:3
-
海关编码:2909500000
-
安全说明:S24/25,S26,S36
-
危险类别码:R20/21/22,R36/37/38
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312+P330,P302+P352+P312+P362+P364,P304+P340+P312,P305+P351+P338+P337+P313,P501
-
危险性描述:H302+H312+H332,H315,H319
SDS
4-Benzyloxyphenyl 4-Hydroxyphenyl Sulfone Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-Benzyloxyphenyl 4-Hydroxyphenyl Sulfone
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 4
Acute toxicity (Oral)
Acute toxicity (Dermal) Category 4
Category 4
Acute toxicity (Inhalation)
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Harmful by inhalation, in contact with skin and if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Avoid breathing.
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-Benzyloxyphenyl 4-Hydroxyphenyl Sulfone
Percent: >98.0%(LC)(T)
CAS Number: 63134-33-8
Chemical Formula: C19H16O4S
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Almost white
Odor: No data available
pH: No data available
Melting point/freezing point:170 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: Soluble in : Methanol
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-Benzyloxyphenyl 4-Hydroxyphenyl Sulfone
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 4
Acute toxicity (Oral)
Acute toxicity (Dermal) Category 4
Category 4
Acute toxicity (Inhalation)
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Harmful by inhalation, in contact with skin and if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Avoid breathing.
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-Benzyloxyphenyl 4-Hydroxyphenyl Sulfone
Percent: >98.0%(LC)(T)
CAS Number: 63134-33-8
Chemical Formula: C19H16O4S
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Almost white
Odor: No data available
pH: No data available
Melting point/freezing point:170 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: Soluble in : Methanol
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
4-羟基-4'-苄氧基二苯砜主要用于合成双(4-羟基苯基)砜O-β- d -葡萄糖醛酸酯,并常作为环氧反应中的反应物。此外,它还被用作环氧树脂的潜在热催化剂。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,2'-磺酰基二苯酚 4,4'-sulfonediphenol 80-09-1 C12H10O4S 250.275 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-benzyloxy-4'-glycidyloxydiphenyl-sulfone 138482-71-0 C22H20O5S 396.464 —— 4-(4-benzyloxyphenylsulfonyl)phenoxypinacolone 172970-73-9 C25H26O5S 438.544 2-[(4-{[4-(苄氧基)苯基]磺酰基}苯氧基)甲基]-2-甲基环氧乙烷 2-methyl-2-[[4-[[4-(phenylmethoxy)phenyl]sulfonyl]phenoxy]methyl]-oxirane 141420-50-0 C23H22O5S 410.491 —— benzene-1,3-dicarboxylic acid 4-{[4-(hydroxyphenyl)sulfonyl]phenyl} ester 392743-70-3 C20H14O7S 398.393 —— benzene-1,3-dicarboxylic acid 4-{[4-(benzyloxy)phenyl]sulfonyl}phenyl benzyl diester 392743-68-9 C34H26O7S 578.642 —— 3-[(methylamino)carbonyl]benzoic acid 4-{[4-(benzyloxy)phenyl]sulfonyl}phenyl diester 392743-72-5 C28H23NO6S 501.56 —— 3-[(methylamino)carbonyl]benzoic acid 4-[4-(hydroxyphenyl)sulfonyl]phenyl ester 392743-73-6 C21H17NO6S 411.435
反应信息
-
作为反应物:描述:4-羟基-4'-苄氧基二苯砜 在 10percent Pd/C 氢气 、 三乙胺 作用下, 以 二氯甲烷 、 氯仿 为溶剂, 20.0 ℃ 、275.8 kPa 条件下, 反应 3.0h, 生成 benzene-1,3-dicarboxylic acid 4-{[4-(hydroxyphenyl)sulfonyl]phenyl} ester参考文献:名称:反应性免疫引发催化聚酯水解的抗体摘要:在寻找用于降解非天然聚合物的生物催化剂时,用半抗原7和11进行反应性免疫制备了能够裂解短的低聚酯以及不溶性聚酯25的催化抗体。发现这些抗体是针对寡聚物的高度特异性和有效的酯酶。三酯24优先通过内切途径水解,但是,使用较高分子量的聚合物25则无法观察到位点特异性。由于物理限制,抗体对不溶性聚合物25的催化效率受到限制。DOI:10.1039/b105412k
-
作为产物:描述:参考文献:名称:ARIENT, JOSEF;DAVIDKOVA, PAVLA;DOSTAL, JOSEF摘要:DOI:
文献信息
-
RECORDING MATERIAL PRODUCED USING NON-PHENOL COMPOUND申请人:NIPPON SODA CO., LTD.公开号:US20150284321A1公开(公告)日:2015-10-08An object of the present invention is to provide a recording material or a recording sheet using, as a color-developing agent, a non-phenol compound that is a safe compound in no danger of corresponding to an endocrine disruptor and is good in color developing performance. The non-phenol compound used in the present invention is at least one selected from the group consisting of compounds represented by the following formulas (I) to (III).
-
Thermosensitive recording material and color developer compound therefor申请人:——公开号:US20040071187A1公开(公告)日:2004-04-15A thermosensitive recording material has a support and a thermosensitive coloring layer formed thereon containing a leuco dye and a color developer capable of inducing color formation in the leuco dye upon application of heat thereto, with the color developer including at least one compound (A) having in a molecule thereof at least two aromatic ring moieties with specific structures, selected from the group consisting of an aromatic ring moiety having at least one carboxyl group and electron-attracting functional group, an aromatic ring moiety having at least one carboxyl group and electron-donating functional group, and an aromatic ring moiety having at least one carboxyl group, free of the electron-attracting and electron-donating functional groups. An aromatic carboxylic acid compound serving as the above-mentioned compound (A) and the producing method thereof are also disclosed.
-
RECORDING MATERIAL COMPRISING DIPHENYL SULFONE DERIVATIVE AND NOVEL DIPHENYL SULFONE DERIVATIVE COMPOUND申请人:NIPPON SODA CO., LTD.公开号:EP1557408A1公开(公告)日:2005-07-27The present invention provides a recording material which has excellent background stability, specifically heat resistance and heat and humidity resistance of the background. The recording material containing a color forming dye is characterized by containing at least one kind of diphenylsulfone derivative represented by the general formula (I): (wherein R1 and R2 each represents a hydrogen atom or a C1 to C6 alkyl group, n represents an integer of 1 to 6, R3 and R4 each represents a halogen atom, a C1 to C6 alkyl group, etc., p and q each represents an integer of 0 to 4, X represents OR5 or NR6R7 wherein R5 represents a C1 to C6 alkyl group, a C1 to C6 hydroxyalkyl group, or a C1 to C6 alkoxy-C1 to C6 alkyl group, and R6 and R7 each represents a hydrogen atom, a C1 to C6 alkyl group, etc).
-
NOVEL DEVELOPER FOR THERMAL RECORDING MEDIA AND THERMAL COMPOSITION MEDIA USING THE SAME申请人:Ruffer, JR. Ronald Q.公开号:US20160089919A1公开(公告)日:2016-03-31The subject invention more specifically discloses compounds which are particularly useful as a developer for thermal recording media. These compounds include p-(p-3-[p-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, m-(p-3-[p-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, o-(p-3-[p-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, p-(p-3-[m-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, and p-(m-3-[o-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol. The present invention also reveals a thermal recording sheet having a thermal recording layer containing a colorless or pale colored dye precursor and a color developer reactable with said dye precursor upon heating to develop a color, wherein said color developer is a compound selected from the group consisting of p-(p-3-[p-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, m-(p-3-[p-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, o-(p-3-[p-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, p-(p-3-[m-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol, and p-(m-3-[o-(p-hydroxyphenoxy)phenyl]ureido}phenoxy)phenol.该专利更具体地揭示了一种作为热记录介质开发剂特别有用的化合物。这些化合物包括p-(p-3-[p-(p-羟基苯氧基)苯基]脲基}苯氧基)酚、m-(p-3-[p-(p-羟基苯氧基)苯基]脲基}苯氧基)酚、o-(p-3-[p-(p-羟基苯氧基)苯基]脲基}苯氧基)酚、p-(p-3-[m-(p-羟基苯氧基)苯基]脲基}苯氧基)酚和p-(m-3-[o-(p-羟基苯氧基)苯基]脲基}苯氧基)酚。本发明还揭示了一种热记录纸张,其具有包含无色或淡色染料前体的热记录层和与所述染料前体在加热时发生反应以形成颜色的颜色开发剂,其中所述颜色开发剂是从以下组合中选取的化合物:p-(p-3-[p-(p-羟基苯氧基)苯基]脲基}苯氧基)酚、m-(p-3-[p-(p-羟基苯氧基)苯基]脲基}苯氧基)酚、o-(p-3-[p-(p-羟基苯氧基)苯基]脲基}苯氧基)酚、p-(p-3-[m-(p-羟基苯氧基)苯基]脲基}苯氧基)酚和p-(m-3-[o-(p-羟基苯氧基)苯基]脲基}苯氧基)酚。
-
[EN] PROCESS FOR THE PRODUCTION OF DIPHENYL SULFONE COMPOUNDS<br/>[FR] PROCESSUS DE FABRICATION DE COMPOSÉS À BASE DE DIPHÉNYLSULFONE申请人:BANDODKAR HEMANT RATANAKAR公开号:WO2014170709A1公开(公告)日:2014-10-23A method for controlled production of mono and diether derivatives of 4,4'-dihydroxydiphenyl sulfone (SDP) namely 4-substituted-4'-hydroxydiphenyl sulfones and 4,4'-disubstituted diphenyl sulfones in high yields by reacting alkyl halide and the like, with 4,4'- dihydroxydiphenyl sulfone. 4-substituted-4'-hydroxydiphenyl sulfone is prepared by reacting equimolar ratio of 4,4'-dihydroxydiphenyl sulfone, alkyl halide and aqueous alkali solution with >80% yield. 4,4'-disubstituted diphenyl sulfone is prepared by reacting one mole of 4,4'- dihydroxydiphenyl sulfone, two moles of alkyl halide and like and two moles of aqueous alkali solution with >80% yield. The method of invention is useful in production of diphenyl sulfone compounds such as 4-allyloxy-4'-hydroxydiphenyl sulfone, 4,4'-diallyloxydiphenyl sulfone, 4-isopropxy-4'-hydroxydiphenyl sulfone, 4,4'-diisopropoxydiphenyl sulfone, 4- benzyloxy-4'-hydroxydiphenyl sulfone, 4,4'-dibenzyloxydiphenyl sulfone etc. This is a commercially feasible green manufacturing process.一种控制生产4,4'-二羟基二苯砜(SDP)的单醚和双醚衍生物的方法,即通过将烷基卤化物等与4,4'-二羟基二苯砜反应,高产率地制备4-取代-4'-羟基二苯砜和4,4'-二取代二苯砜。通过将4,4'-二羟基二苯砜、烷基卤化物和水溶性碱性溶液的等摩尔比反应,可以制备出产率>80%的4-取代-4'-羟基二苯砜。通过将1摩尔的4,4'-二羟基二苯砜、2摩尔的烷基卤化物和类似物以及2摩尔的水溶性碱性溶液反应,可以制备出产率>80%的4,4'-二取代二苯砜。该发明的方法可用于生产二苯砜化合物,例如4-丙烯氧基-4'-羟基二苯砜、4,4'-二丙烯氧基二苯砜、4-异丙氧基-4'-羟基二苯砜、4,4'-二异丙氧基二苯砜、4-苄氧基-4'-羟基二苯砜、4,4'-二苄氧基二苯砜等。这是一种商业可行的绿色制造过程。
表征谱图
-
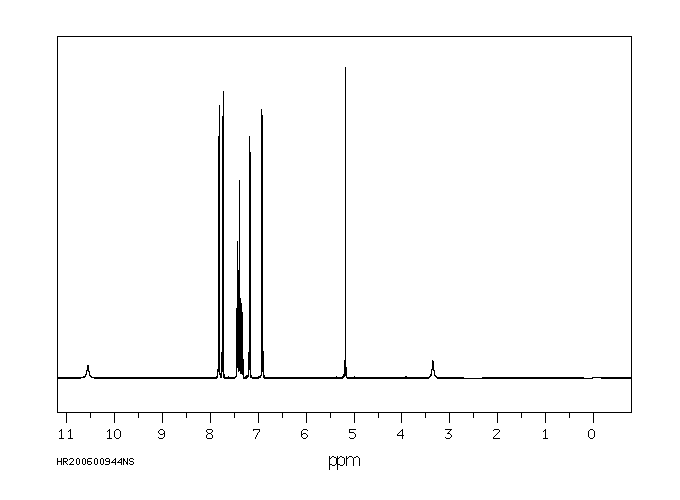
氢谱1HNMR
-
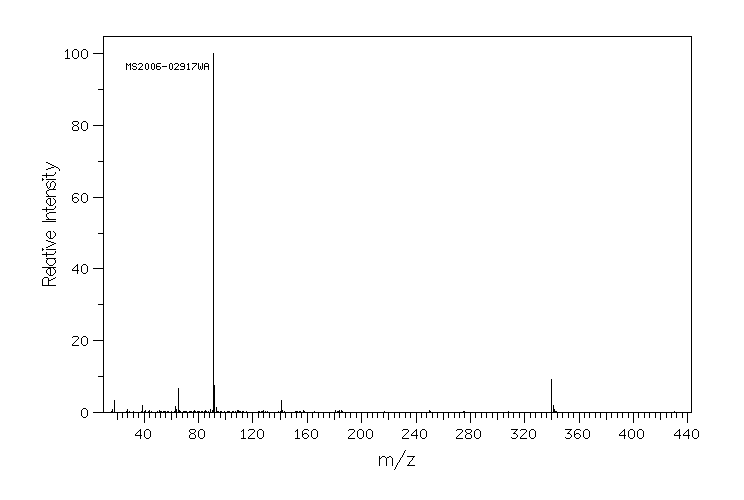
质谱MS
-
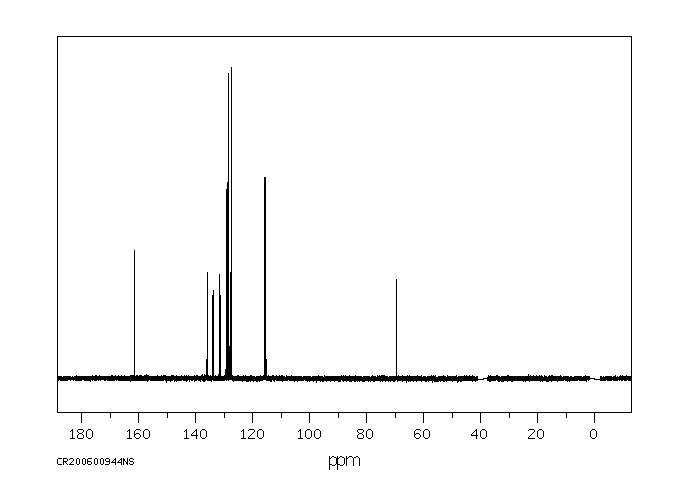
碳谱13CNMR
-
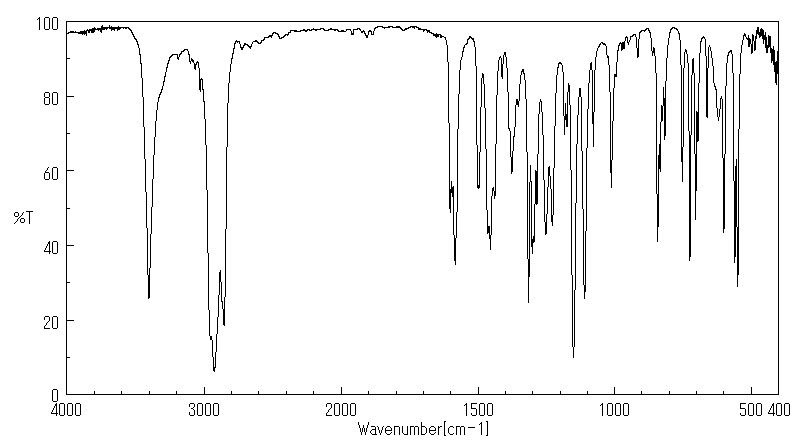
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫