11-苯氧基十一烷酸 | 7170-44-7
中文名称
11-苯氧基十一烷酸
中文别名
11-苯氧基十一酸
英文名称
11-Phenoxyundecanoic acid
英文别名
11-Phenoxy-undecansaeure
CAS
7170-44-7
化学式
C17H26O3
mdl
MFCD00002733
分子量
278.392
InChiKey
FRSQLPPSRJNREN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:77-78 °C(lit.)
-
沸点:217-222 °C3 mm Hg(lit.)
-
密度:1.0352 (rough estimate)
-
溶解度:氯仿:可溶,25mg/mL,澄清(无色至橙色)
计算性质
-
辛醇/水分配系数(LogP):5.2
-
重原子数:20
-
可旋转键数:12
-
环数:1.0
-
sp3杂化的碳原子比例:0.588
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2918990090
SDS
| Name: | 11-Phenoxyundecanoic acid 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 7170-44-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7170-44-7 | 11-phenoxyundecanoic acid, 99% | 99 | 230-520-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 7170-44-7: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: pink
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 217.0 - 222.0 deg C @ 3.00mm
Freezing/Melting Point: 75.00 - 77.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C17H26O3
Molecular Weight: 278.38
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7170-44-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
11-phenoxyundecanoic acid, 99% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 7170-44-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 7170-44-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 7170-44-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-Hydroxy-9-phenoxynonanoic acid 155638-21-4 C15H22O4 266.33 —— (9-bromo-nonyl)-phenyl ether 52176-62-2 C15H23BrO 299.251 —— 3-Hydroxy-5-phenoxypentanoic acid 155638-20-3 C11H14O4 210.23 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-十一烷醇,11-苯氧基- 11-phenoxy-1-undecanol 19097-15-5 C17H28O2 264.408 —— decyloxybenzene 35021-67-1 C16H26O 234.382 —— 1,1,1-trifluoro-12-phenoxydodecan-2-one 492462-83-6 C18H25F3O2 330.391
反应信息
-
作为反应物:描述:11-苯氧基十一烷酸 在 lithium aluminium tetrahydride 作用下, 以 四氢呋喃 为溶剂, 反应 16.0h, 以80%的产率得到1-十一烷醇,11-苯氧基-参考文献:名称:在结核分枝杆菌中开发脂肪酰基-AMP 和脂肪酰基-CoA 连接酶的小分子抑制剂。摘要:结核分枝杆菌( Mtb ) 的脂质代谢依赖 34 种脂肪酸腺苷酸化酶 (FadDs),可分为两类:参与脂质和胆固醇分解代谢的脂肪酰基辅酶 A 连接酶 (FACL) 和长链脂肪酰基-AMP 连接酶 (FAAL) ) 参与Mtb 中发现的许多必需和赋予毒力的脂质的生物合成。许多 FACL 的精确生化作用仍不清楚,而功能非冗余的 FAAL 则更好理解。这里,我们描述的5'-系统调查ö - [ ñ - (链烷酰基)氨磺酰基]腺苷(烷酰基一denosine米ONO小号ulfamate, alkanoyl-AMS) 类似物作为潜在的多靶点 FadD 抑制剂,因为它们具有抗结核活性和对代表性 FAAL 和 FACL 酶的生化选择性。我们鉴定了几种有效的化合物,包括 12-叠氮十二烷酰基-AMS 28、11-苯氧基十一烷酰基-AMS 32和壬氧基乙酰基-AMS 36,其对结核分枝杆菌的最小抑制浓度 (MIC)范围为DOI:10.1016/j.ejmech.2020.112408
-
作为产物:描述:参考文献:名称:Modulation of vesicular properties by variation of shapes of bolaform counter ions in hybrid-ion paried surfactants摘要:合成了四种新的囊泡形成亲波拉/两亲离子对;这种混合系统中的亲水性形状强烈控制其囊泡特性。DOI:10.1039/cc9960001283
文献信息
-
Synthesis and biological evaluation of crown ether acyl derivatives作者:Martín Febles、Sofía Montalvão、Guillermo Díaz Crespín、Manuel Norte、José M. Padrón、Päivi Tammela、José J. Fernández、Antonio Hernández DaranasDOI:10.1016/j.bmcl.2016.09.066日期:2016.11A set of crown ethyl acyl derivatives based on 18-crown-6 moiety was synthesized and evaluated for biological activity. In vitro antiproliferative profiling demonstrated significant activities against HBL-100, HeLa, SW1573 and WiDr human cell lines. The most active compound exhibited GI50 values in the range of 3.7-5.6μM. Antimicrobial evaluation showed that three polyaromatic compounds were active
-
Method of treating lipidemia with aryloxyalkylaminobenzoic acids and
-
Structure-Activity Relationships of C1 and C6 Side Chains of Zaragozic Acid A Derivatives作者:Mitree M. Ponpipom、Narindar N. Girotra、Robert L. Bugianesi、Cathleen D. Roberts、Gregory D. Berger、Robert M. Burk、Robert W. Marquis、William H. Parsons、Kenneth F. BartizalDOI:10.1021/jm00049a022日期:1994.11esters. In the preparation of C6 ethers, C4 and C4,6 bisethers were also isolated; their relative activity is: C6 > C4 > C4,6. These C6 long-chain derivatives are subnanomolar squalene synthase inhibitors; they are, however, only weakly active in inhibiting hepatic cholesterol synthesis in mice. The C6 short-chain derivatives are much less active in vitro, but they all have improved oral activity in系统地修改了一种有效的角鲨烯合酶抑制剂zaragozic A的C6酰基侧链,以改善其生物学活性。将C6侧链简化为辛酸酯具有有害作用。增加线性链长度可提高体外活性,直至十四烷酸酯为止。ω-苯氧基比ω-苯基更好的活性增强剂。制备了许多C6氨基甲酸酯,醚和碳酸酯,发现它们具有与C6酯相似的活性。在制备C6醚时,还分离出C4和C4,6双醚。它们的相对活性为:C6> C4> C4,6。这些C6长链衍生物是亚纳摩尔角鲨烯合酶抑制剂。然而,它们仅在抑制小鼠肝胆固醇合成中具有弱活性。C6短链衍生物在体外的活性低得多,但它们在小鼠中的口服活性均得到改善。正丁酰基类似物的C1烷基侧链(ED50为4.5 mg / kg)的修饰不能进一步提高po活性。这些C6长链衍生物中的许多也是体外有效的抗真菌剂。
-
4-DIMETHYLAMINOBUTYRIC ACID DERIVATIVES申请人:Andjelkovic Mirjana公开号:US20090270505A1公开(公告)日:2009-10-29This invention relates to novel 4-dimethylaminobutyric acid derivatives of the formula wherein A 1 , A 2 , R 1 , m and n are as defined in the description and in the claims, as well as pharmaceutically acceptable salts thereof. These compounds inhibit carnitine palmitoyl transferase (CPT) activity, in particular CPT2 activity, and can be used as medicaments in methods for the treatment of diseases modulated by CPT2 inhibitors.
-
Colorant compounds申请人:Banning H. Jeffrey公开号:US20060020141A1公开(公告)日:2006-01-26Compounds of the formula wherein M is either (1) a metal ion having a positive charge of +y wherein y is an integer which is at least 2, said metal ion being capable of forming a compound with at least two chromogen moieties, or (2) a metal-containing moiety capable of forming a compound with at least two chromogen moieties, z is an integer representing the number of chromogen moieties associated with the metal and is at least 2, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , a, b, c, d, Y, and z are as defined herein, Q − is a COO − group or a SO 3 — group, A is an organic anion, and CA is either a hydrogen atom or a cation associated with all but one of the Q − groups.
表征谱图
-
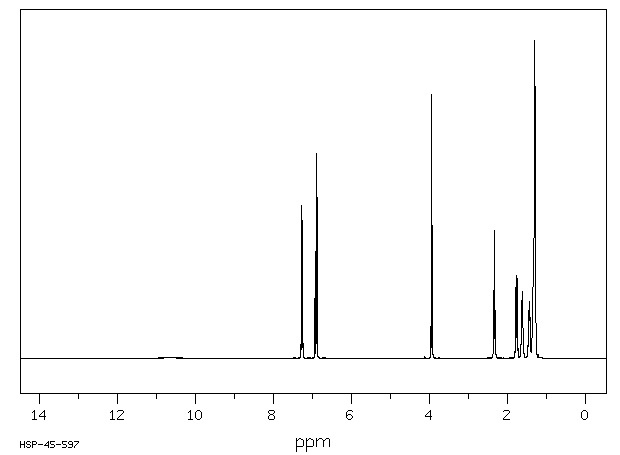
氢谱1HNMR
-
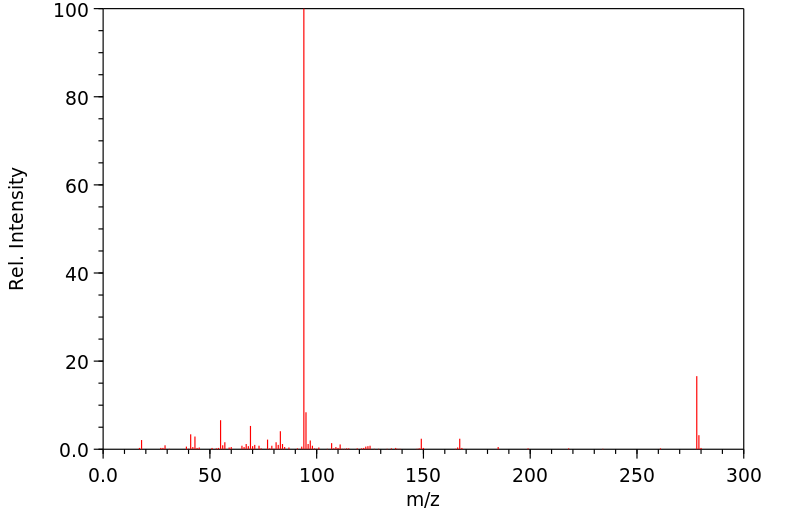
质谱MS
-
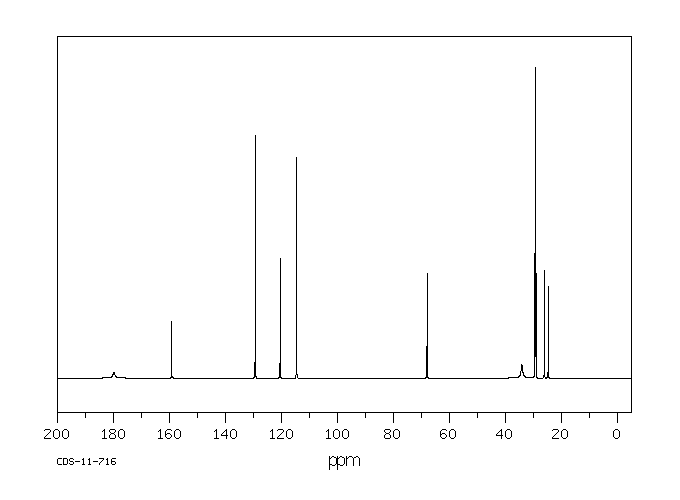
碳谱13CNMR
-
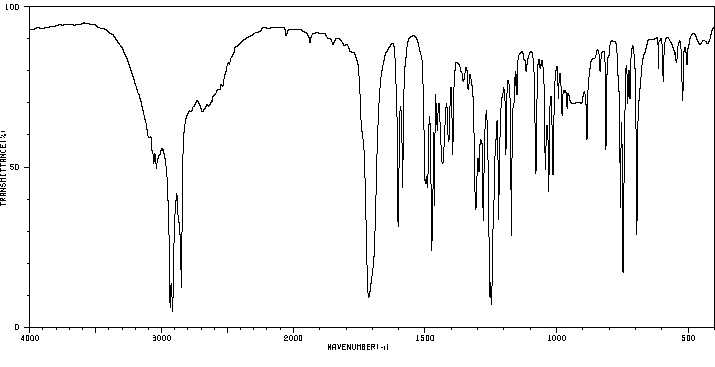
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯