N-甲基-3-氯丙胺盐酸盐 | 97145-88-5
物质功能分类
中文名称
N-甲基-3-氯丙胺盐酸盐
中文别名
——
英文名称
N-methyl-3-chloropropylamine hydrochloride
英文别名
3-chloro-N-methylpropan-1-amine hydrochloride;3-chloropropyl-N-methylamine hydrochloride;3-chloro-N-methylpropan-1-amine;hydrochloride
CAS
97145-88-5
化学式
C4H10ClN*ClH
mdl
MFCD00144935
分子量
144.044
InChiKey
SHBWHBGVZIDIQY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:105°C
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):0.53
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12
-
氢给体数:2
-
氢受体数:1
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2921199090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
N-Methyl-3-chloropropylamine Hydrochloride
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N-Methyl-3-chloropropylamine Hydrochloride
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N-Methyl-3-chloropropylamine Hydrochloride
Percent: >99.0%(T)
CAS Number: 97145-88-5
Synonyms: 3-(Methylamino)propyl Chloride Hydrochloride
Chemical Formula: C4H10ClN·HCl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
Melting point/freezing point:105°C
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] No data available
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N-Methyl-3-chloropropylamine Hydrochloride
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N-Methyl-3-chloropropylamine Hydrochloride
Percent: >99.0%(T)
CAS Number: 97145-88-5
Synonyms: 3-(Methylamino)propyl Chloride Hydrochloride
Chemical Formula: C4H10ClN·HCl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
Melting point/freezing point:105°C
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] No data available
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:N-甲基-3-氯丙胺盐酸盐 在 sodium iodide 作用下, 以 乙腈 为溶剂, 反应 0.5h, 以95%的产率得到3-iodo-N-methylpropan-1-amine hydrochloride参考文献:名称:[EN] PROSTAGLANDIN DERIVATIVES
[FR] DERIVES DE PROSTAGLANDINE摘要:公开号:WO2007000641A3 -
作为产物:描述:参考文献:名称:싸이클로펜타디엔일 유도체 중간체 및 상기 싸이클로펜타디엔일 유도체 제조방법摘要:本发明涉及用于原子层沉积(ALD:Atomic Layer Deposition)或化学气相沉积(Chemical Vapor Deposition)工艺中使用的自限制反应(Self-limiting reaction)的金属前体(Metal Precursor)的构成,其中金属前体是化学式3的环戊二烯甲基胺(以下简称CpEMA)或化学式4的环戊二烯甲基丙胺(以下简称CpPMA)的配体化合物。本发明涉及一种用于制备CpEMA或CpPMA的新中间体的方法,该中间体是化学式1的环戊二烯甲基胺乙酸盐或化学式2的环戊二烯甲基丙胺乙酸盐,并且将化学式1或化学式2的化合物作为中间体,以制备化学式3和化学式4的化合物。本发明提供了一种利用新型中间体简便易行地制备高纯度的环戊二烯甲基衍生物,以构成用于原子层沉积(ALD:Atomic Layer Deposition)或化学气相沉积(Chemical Vapor Deposition)工艺中使用的前体(Precursor)中的自限制反应(Self-limiting reaction)的前体。公开号:KR20180054180A
文献信息
-
Novel Analogues of Istaroxime, a Potent Inhibitor of Na<sup>+</sup>,K<sup>+</sup>-ATPase: Synthesis and Structure−Activity Relationship作者:Mauro Gobbini、Silvia Armaroli、Leonardo Banfi、Alessandra Benicchio、Giulio Carzana、Giorgio Fedrizzi、Patrizia Ferrari、Giuseppe Giacalone、Michele Giubileo、Giuseppe Marazzi、Rosella Micheletti、Barbara Moro、Marco Pozzi、Piero Enrico Scotti、Marco Torri、Alberto CerriDOI:10.1021/jm800257s日期:2008.8.1We report the synthesis and biological properties of novel inhibitors of the Na(+),K(+)-ATPase as positive inotropic compounds. Following our previously described model from which Istaroxime was generated, the 5alpha,14alpha-androstane skeleton was used as a scaffold to study the space around the basic chain of our lead compound. Some compounds demonstrated higher potencies than Istaroxime on the receptor
-
AMINO DERIVATIVES OF ANDROSTANES AND ANDROSTENES AS MEDICAMENTS FOR CARDIOVASCULAR DISORDERS申请人:Cerri Alberto公开号:US20110053902A1公开(公告)日:2011-03-03Compounds of formula (I) wherein: the groups are as defined in the description, are useful for the preparation of medicaments for the treatment of cardiovascular disorders, in particular heart failure and hypertension. The compounds are inhibitors of the enzymatic activity of the Na + , K + -ATPase. Said compounds are used for the preparation of a medicament for the treatment of a disease caused by the hypertensive effects of endogenous ouabain, such as renal failure progression in autosomal dominant polycystic renal disease (ADPKD), preeclamptic hypertension and proteinuria and renal failure progression in patients with adducin polymorphisms.
-
[EN] ANTI-NEOPLASTIC COMPOUNDS, COMPOSITIONS AND METHODS<br/>[FR] COMPOSÉS ANTINÉOPLASIQUES, COMPOSITIONS ET PROCÉDÉS
-
DIAGNOSIS, TREATMENT AND PREVENTION OF NEUROTENSIN RECEPTOR-RELATED CONDITIONS申请人:Friedrich-Alexander-Universität Erlangen-Nürnberg公开号:EP3279197A1公开(公告)日:2018-02-07The present invention describes a compound of formula (I) which can be used in the diagnosis, treatment or prevention of neurotensin receptor-related conditions such as tumors and hematological malignancies.本发明描述了一种化合物,其化学式为(I),可用于诊断、治疗或预防与神经肽受体相关的疾病,如肿瘤和血液恶性肿瘤。
-
Synthesis and Antiproliferative Activity of Marine Bromotyrosine Purpurealidin I and Its Derivatives作者:Chinmay Bhat、Polina Ilina、Irene Tilli、Manuela Voráčová、Tanja Bruun、Victoria Barba、Nives Hribernik、Katja-Emilia Lillsunde、Eero Mäki-Lohiluoma、Tobias Rüffer、Heinrich Lang、Jari Yli-Kauhaluoma、Paula Kiuru、Päivi TammelaDOI:10.3390/md16120481日期:——Purpurealidin I (1) showed no selectivity but its simplified pyridin-2-yl derivative (36) had the best improvement in selectivity (Selectivity index 4.1). This shows that the marine bromotyrosines are promising scaffolds for developing cytotoxic agents and the full understanding of the elements of their SAR and improving the selectivity requires further optimization of simplified bromotyrosine derivatives.
表征谱图
-
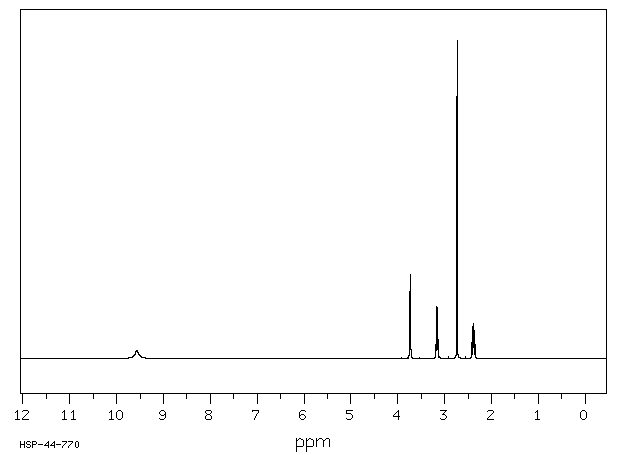
氢谱1HNMR
-
质谱MS
-
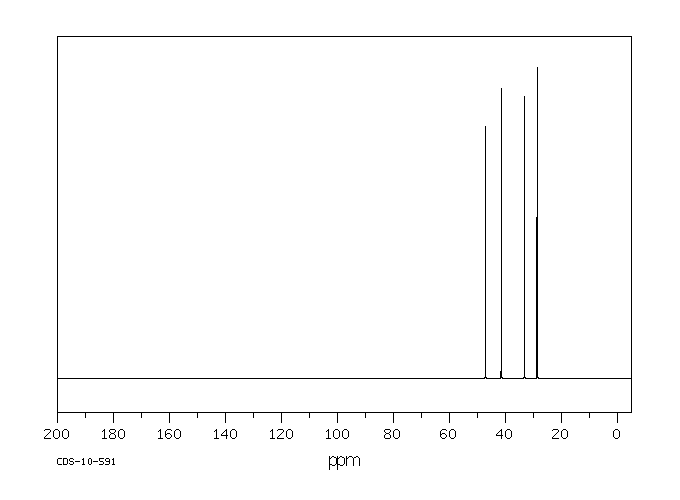
碳谱13CNMR
-
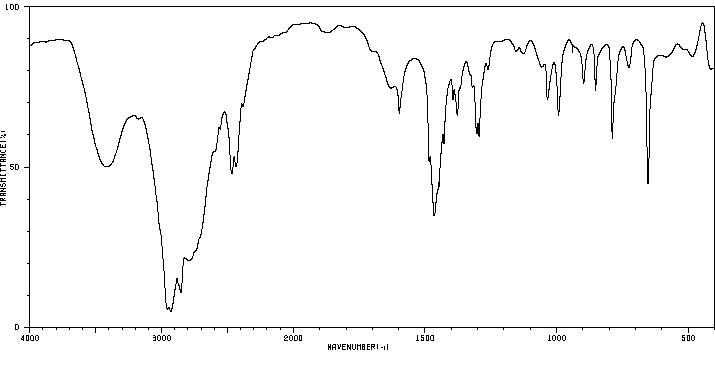
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷