5-氨基-1-萘酚-3-磺酸 | 489-78-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:1.627±0.06 g/cm3(Predicted)
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:109
-
氢给体数:3
-
氢受体数:5
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2922299090
SDS
模块 1. 化学品
产品名称: 5-Amino-1-naphthol-3-sulfonic Acid Hydrate
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5-氨基-1-萘酚-3-磺酸水合物
百分比: >97.0%(T)
CAS编码: 489-78-1
俗名: 8-Amino-4-hydroxy-2-naphthalenesulfonic Acid Hydrate , M Acid Hydrate
5-氨基-1-萘酚-3-磺酸水合物 修改号码:6
模块 3. 成分/组成信息
分子式:
C10H9NO4S·xH2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
5-氨基-1-萘酚-3-磺酸水合物 修改号码:6
模块 9. 理化特性
颜色: 黄红色-深紫色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
5-氨基-1-萘酚-3-磺酸水合物 修改号码:6
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
暂无相关描述。
用途暂无相关描述。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-8-萘酚-6-磺酸 6-amino-4-hydroxy-2-naphthalenesulfonic acid 90-51-7 C10H9NO4S 239.252 7-氨基-4-羟基-2-萘磺酸 1-hydroxy-6-amino-3-naphthalenesulfonic acid 87-02-5 C10H9NO4S 239.252 5-氨基-1-萘磺酸 1-aminonaphthalene-5-sulfonic acid 84-89-9 C10H9NO3S 223.252 8-硝基萘-2-磺酸 1-nitro-7-naphthalenesulfonic acid 18425-74-6 C10H7NO5S 253.235 —— 5-Aminonaphthalene-1,3-disulfonic acid 13306-42-8 C10H9NO6S2 303.317 5-氨基-1,3,6-萘三磺酸 5-amino-naphthalene-1,3,6-trisulfonic acid 67900-43-0 C10H9NO9S3 383.381 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 8-Hydrazinyl-4-hydroxynaphthalene-2-sulfonic acid 924277-31-6 C10H10N2O4S 254.266 —— 8-[(2-Boronophenyl)methylamino]-4-hydroxynaphthalene-2-sulfonic acid 1351863-08-5 C17H16BNO6S 373.194
文献信息
-
[EN] AZO DYES<br/>[FR] COLORANTS AZOÏQUES申请人:KEMIRA OYJ公开号:WO2012072634A1公开(公告)日:2012-06-07The present invention relates to new azo dyes, a process for their preparation, and their use for dyeing or printing fibrous materials, to produce materials with brownish shades.本发明涉及新的偶氮染料,其制备过程,以及它们用于染色或印花纤维材料以产生棕色调材料的用途。
-
Process for Printing an Image, Composition and Compound申请人:Monahan Lilian公开号:US20110014435A1公开(公告)日:2011-01-20A process for printing an image on a substrate, the process comprising applying to the substrate an ink composition which comprises a liquid medium and a compound selected from the group consisting of compounds of Formula (1 I ), compounds of Formula (1 II ), compounds of Formula (1 III ) and compounds of Formula (1 IV ): wherein: A and D each independently represent optionally substituted aryl or optionally substituted heteroaryl; E represents optionally substituted pyrazolyl; Z represents H, halogen, nitro, cyano, hydroxy, amino, carboxy, optionally substituted alkyl, optionally substituted alkoxy or optionally substituted aryloxy; and p is an integer from 0 to 5. Preferably, the printing process is ink jet printing. Also provided are compounds of Formula (1 I ), (1 II ), (1 III ) and (1 IV ) and ink compositions comprising the same.
-
Using Quenching Kinetics and Thermodynamics of Amino-Fluorophores as Empirical Tools for Predicting Boronic Acid Sensors Suitable for Use in Physiological Conditions作者:Nicholas McGregor、Christophe Pardin、W. G. SkeneDOI:10.1071/ch11297日期:——
A series of water-soluble 1-amino-naphthalenes and 2-amino-fluorenes are prepared. These serve as model fluorophores for measuring the thermodynamics and kinetics of fluorescence quenching with phenylboronic acids and aliphatic amines. Steady-state and time-resolved fluorescence quenching kinetics are investigated using the Stern–Volmer method. Diffusion limited quenching constants and exergonic thermodynamics of electron transfer are derived for the 5-amino-1-napthol and 2-aminofluorene derivatives with phenylboronic acid and/or an aliphatic imine. No quenching and endergonic thermodynamics or electron transfer are observed for 5-sulfonamide, 5-sulfonic acid, or 5-hydroxy-7-sulfonic acid aminonaphthalene derivatives. Boronic acid sensors synthesized from these aminofluorophores by reductive amination with 2-formylphenylboronic acid undergo fluorescence revival in the presence of saccharides only when the fluorophore demonstrates diffusion limited quenching kinetics and exergonic thermodynamics of electron transfer with the boronic acid or imine quenchers. Thus, these two properties are suitable empirical tools for predicting saccharide-induced fluorescence revival of boronic acid sensors.
制备了一系列水溶性 1-氨基萘和 2-氨基芴。这些荧光团可作为模型荧光团,用于测量苯硼酸和脂肪族胺的荧光淬灭热力学和动力学。使用 Stern-Volmer 方法研究了稳态和时间分辨荧光淬灭动力学。得出了 5-氨基-1-萘酚和 2-氨基芴衍生物与苯硼酸和/或脂肪族亚胺的扩散受限淬灭常数和电子转移的外功热力学。对于 5-磺酰胺、5-磺酸或 5-羟基-7-磺酸氨基萘衍生物,没有观察到淬灭和内生热力学或电子转移。通过与 2-甲酰基苯硼酸进行还原胺化反应,由这些氨基荧光团合成的硼酸传感器只有在荧光团显示出扩散受限的淬灭动力学以及与硼酸或亚胺淬灭剂进行电子转移的放生热力学时,才会在糖类存在的情况下发生荧光恢复。因此,这两个特性是预测糖诱导的硼酸传感器荧光复苏的合适经验工具。 -
Azo Compound Or Salt Thereof, And Dye-Based Polarizing Film And Dye-Based Polarizing Plate Containing Same申请人:Nippon Kayaku Kabushiki Kaisha公开号:US20220010139A1公开(公告)日:2022-01-13An azo compound represented by the following formula (1) or a salt thereof, Wherein A 1 represents a naphthyl group which may have a substituent; A 2 , A 3 , and A 4 each independently represent a phenyl group which may have a substituent or a naphthyl group which may have a substituent; R 1 represents a hydrogen atom, a hydroxy group, a C1-4 alkoxy group, or a substituted or unsubstituted amino group; m represents an integer of 0 to 5; M represents a hydrogen atom or ion, a metal ion, or an ammonium ion; n represents 1 or 2; k represents 0 or 1; and each hydrogen atom on ring a and ring b may be substituted with the substituent R 1 or substituent SO 3 M.
-
Sulfonamide based antimicrobial reactive dyes: A study of their synthesis, fastness and antimicrobial activity作者:Shafia Sagheer、Abdul Jabbar、Muhammad Kashif Pervez、Kiran Rani、Ambreen、Saadia RiazDOI:10.1016/j.molstruc.2021.131837日期:2022.2cellulosic fibre dyeing, and antimicrobial finishing are two energy extensive processes. In the present study, an attempt has been made to combine these two processes, which would in turn save time, energy, and cost. In this regard, a new series of reactive dyes (SM1-SM6) has been synthesized using antimicrobial sulfonamide components as dyestuff intermediates. The resultant dyes were characterized
表征谱图
-
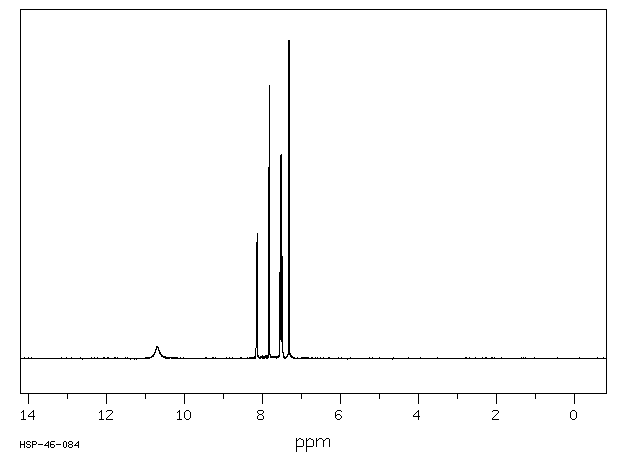
氢谱1HNMR
-
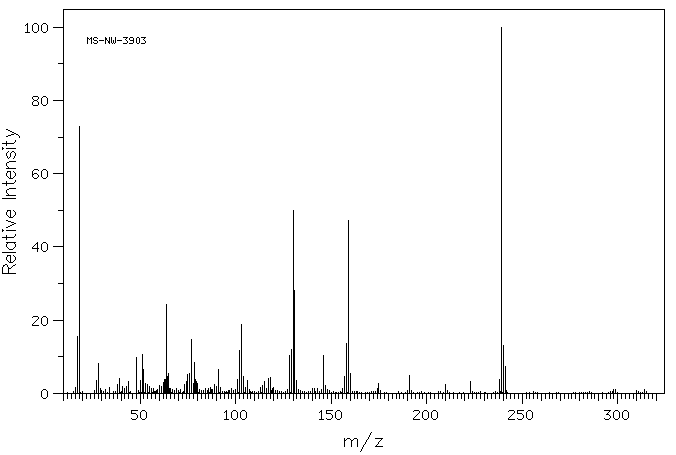
质谱MS
-
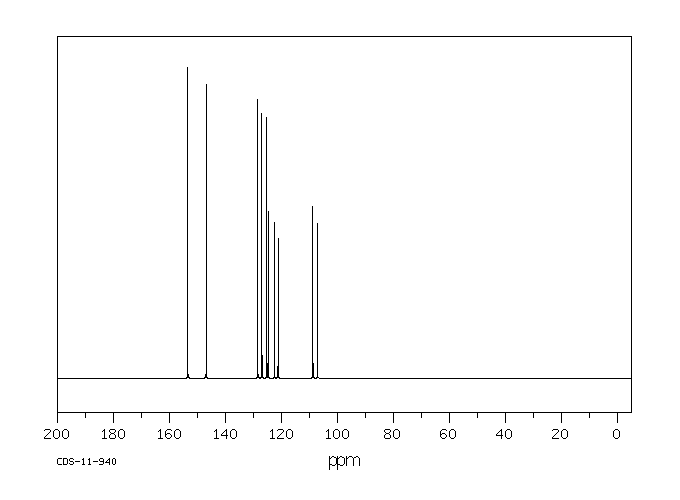
碳谱13CNMR
-
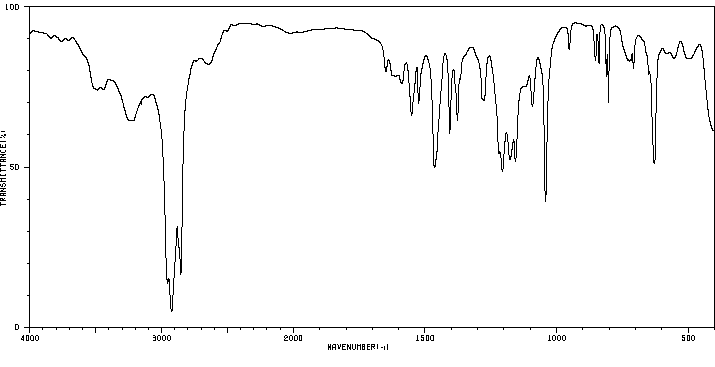
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息