1-(3-甲基丁基)环戊烯 | 37689-15-9
中文名称
1-(3-甲基丁基)环戊烯
中文别名
——
英文名称
1-isopentylcyclopent-1-ene
英文别名
isopentylcyclopentene;1-isopentyl-cyclopentene;1-Isopentyl-cyclopenten;1-isopentyl-1-cyclopentene;Isoamyl-cyclopenten;Cyclopentene, 1-(3-methylbutyl)-;1-(3-methylbutyl)cyclopentene
CAS
37689-15-9
化学式
C10H18
mdl
——
分子量
138.253
InChiKey
LDBWEVZRYRUOOH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1001;1005;984;1001;1005;977;981.6;977;982;984
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:10
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Schuikin; Tscherkaschin, Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1957, p. 1256; engl. Ausg. S. 1278摘要:DOI:
-
作为产物:描述:参考文献:名称:Chavanne; Becker, Bulletin des Societes Chimiques Belges, 1927, vol. 36, p. 594摘要:DOI:
文献信息
-
GaCl<sub>3</sub>-Catalyzed Ring-Opening Carbonyl–Olefin Metathesis作者:Haley Albright、Hannah L. Vonesh、Marc R. Becker、Brandon W. Alexander、Jacob R. Ludwig、Ren A. Wiscons、Corinna S. SchindlerDOI:10.1021/acs.orglett.8b02086日期:2018.8.17The development of a Lewis acid-catalyzed ring-opening cross-metathesis reaction which enables selective access to acyclic, unsaturated ketones as the carbonyl-olefin metathesis products is described. While catalytic amounts of FeCl3 were previously identified as optimal to catalyze ring-closing metathesis reactions, the complementary ring-opening metathesis between cyclic alkenes and carbonyl functionalities
-
Biernacki,W. et al., Roczniki Chemii, 1976, vol. 50, p. 895 - 901作者:Biernacki,W. et al.DOI:——日期:——
-
Synthesis of Iso-amylcyclopentane.作者:John McArthur HarrisDOI:10.1021/ja01383a503日期:1929.8
表征谱图
-
氢谱1HNMR
-
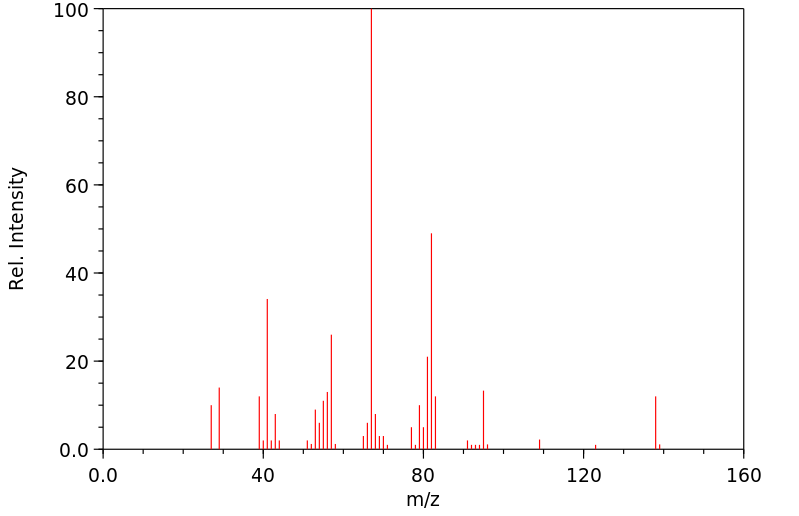
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-