5-甲基-2-己烯 | 53566-37-3
中文名称
5-甲基-2-己烯
中文别名
——
英文名称
5-methyl-2-hexyne
英文别名
5-methylhex-2-yne;5-methyl-hex-2-yne;5-Methyl-hex-2-in;Methyl-isobutyl-acetylen;5-Methyl-2-hexin;5-Methylhex-2-in;2-Hexyne, 5-methyl-
CAS
53566-37-3
化学式
C7H12
mdl
MFCD00041618
分子量
96.1723
InChiKey
SVGAHRUSRQTQES-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-92.9°C
-
沸点:102°C
-
密度:0,738 g/cm3
-
保留指数:701
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-甲基-1-戊炔 4-methylpentyne 7154-75-8 C6H10 82.1454
反应信息
-
作为反应物:参考文献:名称:D'yakonov,I.A. et al., Journal of Organic Chemistry USSR (English Translation), 1969, vol. 5, # 6, p. 1013 - 1018摘要:DOI:
-
作为产物:描述:参考文献:名称:Alkylidenecarbenes from acyclic vinyl bromides and potassium tert-butoxide摘要:DOI:10.1021/jo00867a001
文献信息
-
Gold(<scp>i</scp>) catalysed regio- and stereoselective intermolecular hydroamination of internal alkynes: towards functionalised azoles作者:Christophe Michon、Joachim Gilbert、Xavier Trivelli、Fady Nahra、Catherine S. J. Cazin、Francine Agbossou-Niedercorn、Steven P. NolanDOI:10.1039/c9ob00587k日期:——Gold(I) catalysed regio- and stereoselective intermolecular hydroamination of internal alkynes was developed for the effective synthesis of a series of (Z)-functionalised vinylazoles under solvent free conditions. The catalytic hydrogenation of the resulting enamines leads to substituted saturated azoles in good yields.
-
A Facile Approach to the Synthesis of 3-Acylisoxazole Derivatives with Reusable Solid Acid Catalysts作者:Ken-ichi Itoh、Mamiko Hayakawa、Rina Abe、Shinji Takahashi、Kenta Hasegawa、Tadashi AoyamaDOI:10.1055/a-1581-0235日期:2021.12gel-supported sodium hydrogen sulfate (NaHSO4/SiO2) or Amberlyst 15 as solid acid catalyst, and then the corresponding 3-acylisoxaszoles were obtained by reacting with alkynes via the 1,3-dipolar [3+2] cycloaddition. These heterogeneous catalysts are easily separable from the reaction mixture and reused. This synthetic method provides a facile, efficient, and reusable production of 3-acylisoxazoles.
-
Petrow; Werenzowa; Koklejewa, Zhurnal Obshchei Khimii, 1941, vol. 11, p. 1097作者:Petrow、Werenzowa、KoklejewaDOI:——日期:——
-
Pomerantz et al., Journal of Research of the National Bureau of Standards (United States), 1954, vol. 52, p. 59,60作者:Pomerantz et al.DOI:——日期:——
-
Selective hydroesterification of alkynes to mono- or diesters作者:Howard Alper、Bertrand Despeyroux、James B. WoellDOI:10.1016/s0040-4039(00)94174-1日期:1983.1
表征谱图
-
氢谱1HNMR
-
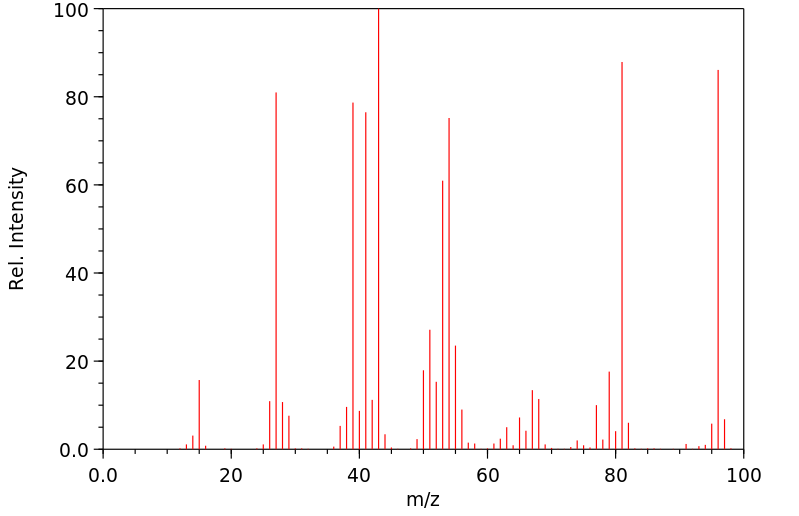
质谱MS
-
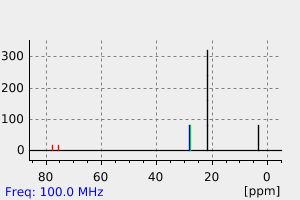
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-