2-甲基-5-辛炔-4-醇 | 60657-70-7
中文名称
2-甲基-5-辛炔-4-醇
中文别名
——
英文名称
2-methyl-5-octyne-4-ol
英文别名
2-methyl-5-octyn-4-ol;2-methyloct-5-yn-4-ol
CAS
60657-70-7
化学式
C9H16O
mdl
MFCD00041690
分子量
140.225
InChiKey
YBGQRUFRRPFNKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:0,855 g/cm3
-
闪点:78°C
-
稳定性/保质期:
避免接触氧化物
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:10
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.777
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
储存条件:在密封的贮藏器中,并将其存放在阴凉、干燥的地方。
SDS
反应信息
-
作为反应物:参考文献:名称:Solvent-modulated Pd/C-catalyzed deprotection of silyl ethers and chemoselective hydrogenation摘要:Recently we have reported undesirable and frequent deprotection of the TBDMS protective group of a variety of hydroxyl functions occurred under neutral and mild hydrogenation conditions using 10% Pd/C in MeOH. The deprotection of silyl ethers is susceptible to significant solvent effect. TBDMS and TES protecting groups were selectively cleaved in the presence of acid-sensitive functional groups such as TIPS ether, TBDPS ether and dimethyl acetal under hydrogenation condition using 10% Pd/C in MeOH. In contrast, chemoselective hydrogenation of reducible functional groups such as acetylene, olefin and benzyl ether, proceeds in the presence of TBDMS or TES ethers in AcOEt or MeCN. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2004.05.098
文献信息
-
<i>trans</i> ‐Hydroboration of Propargyl Alcohol Derivatives and Related Substrates作者:Lauren E. Longobardi、Alois FürstnerDOI:10.1002/chem.201902228日期:2019.8in good to excellent levels of regio‐ as well as stereoselectivity, provided that the triple bond bears one linear and one singly‐branched substituent. In such cases, the reaction follows an unusual trans‐addition mode and places the boron entity distal to the branching point. The resulting alkenyl boronates, which are difficult to make otherwise, can be engaged in numerous enabling downstream processes
-
Rapid Assembly of Structurally Defined and Highly Functionalized Conjugated Dienes via Tethered Enyne Metathesis作者:Qingwei YaoDOI:10.1021/ol016026s日期:2001.6.1[reaction: see text] Conjugated dienes are versatile building blocks in organic synthesis, and the development of new methods for their synthesis remains an important topic in modern synthetic organic chemistry. We describe here an expedient synthesis of highly functionalized conjugated dienes through sequential silicon-tethered ring-closing enyne metathesis mediated by Grubbs' Ru carbene catalysts
-
Luo, Tuoping; Schreiber, Stuart L., Journal of the American Chemical Society, 2009, vol. 131, p. 5667 - 5674作者:Luo, Tuoping、Schreiber, Stuart L.DOI:——日期:——
-
Direct Access to Allenylphosphine Oxides via a Metal Free Coupling of Propargylic Substrates with P(O)H Compounds作者:Chun-Hua Yang、Huihui Fan、Huimin Li、Shenyin Hou、Xiangkun Sun、Donghao Luo、Yinchao Zhang、Zhantao Yang、Junbiao ChangDOI:10.1021/acs.orglett.9b03645日期:2019.12.6A direct and convenient approach for the coupling of propargylic substrates with diphenylphosphine oxide in the presence of Tf2O and 2,6-lutidine has been developed. The method provides a general approach for the construction of attractive allenylphosphoryl skeletons with high atom and step economy under metal free conditions.
-
Complex α-Pyrones Synthesized by a Gold-Catalyzed Coupling Reaction作者:Tuoping Luo、Stuart L. SchreiberDOI:10.1002/anie.200703276日期:2007.11.5
表征谱图
-
氢谱1HNMR
-
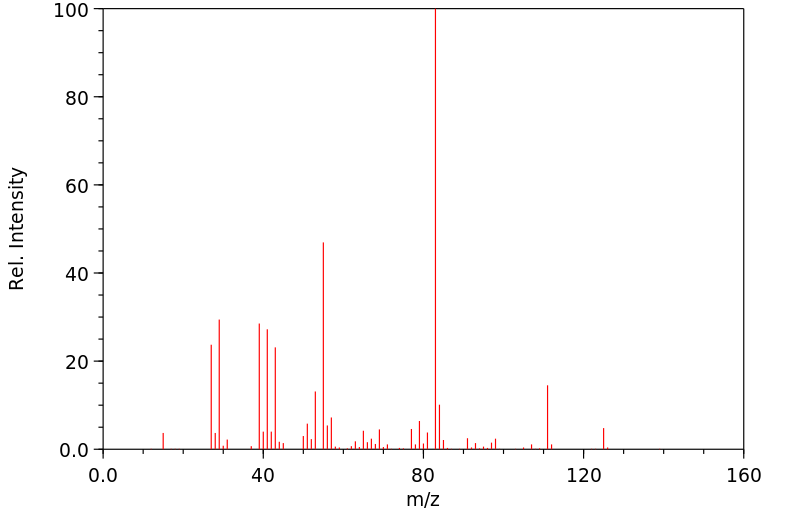
质谱MS
-
碳谱13CNMR
-
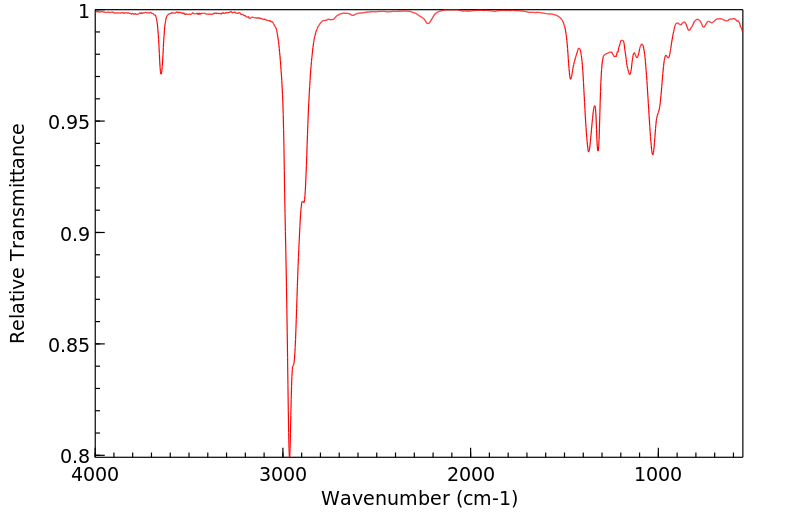
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷