3-甲基-苯并[a]蒽 | 2498-75-1
中文名称
3-甲基-苯并[a]蒽
中文别名
——
英文名称
3-methylbenz[a]anthracene
CAS
2498-75-1
化学式
C19H14
mdl
——
分子量
242.32
InChiKey
VYZKVUIQCRWXJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:156.5°C
-
沸点:452.14°C (rough estimate)
-
密度:1.1011 (estimate)
-
保留指数:2580;417.5
-
稳定性/保质期:
存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):6.4
-
重原子数:19
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,6-二甲基萘 2.6-dimethylnaphthalene 581-42-0 C12H12 156.227 苯并[a]蒽 benz[a]anthracene 56-55-3 C18H12 228.293 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,7,12-trimethylbenzanthracene 35187-23-6 C21H18 270.374
反应信息
-
作为反应物:描述:参考文献:名称:55.多环芳烃。第九部分 1:2-苯并蒽的甲基和异丙基同系物的合成摘要:DOI:10.1039/jr9320000456
-
作为产物:描述:参考文献:名称:55.多环芳烃。第九部分 1:2-苯并蒽的甲基和异丙基同系物的合成摘要:DOI:10.1039/jr9320000456
文献信息
-
Raney nickel reductions—VIII作者:N.B. Desai、K. VenkataramanDOI:10.1016/0040-4020(59)80023-5日期:1959.11:2-Benzanthracene and the 3′-methyl derivative have been prepared by Raney nickel reduction of the sulphuric esters of the leuco derivatives of 1:2-benzanthraquinone and 4′-chloro-3′-methyl-1:2-benzanthraquinone, followed by dehydrogenation. 3-Hydroxy-1:2-benzanthraquinone was methylated in the 4-position by formaldehyde, sodium hydrosulphite and sodium hydroxide solution (the Marschalk reaction)
-
Substituent Effects in Benz[<i>a</i>]anthracene Carbocations: A Stable Ion, Electrophilic Substitution (Nitration, Bromination), and DFT Study作者:Kenneth K. Laali、Maria A. Arrica、Takao Okazaki、Ronald G. HarveyDOI:10.1021/jo070936r日期:2007.8.31computed relative energies by DFT. Charge delocalization paths in the resulting carbocations were deduced based on the magnitude of Δδ13C values. For the thermodynamically more stable C-12 protonated carbocations, the charge delocalization path is analogous to those derived based on computed NPA charges for the benzylic carbocations formed by 1,2-epoxide (bay-region) and 5,6-epoxide (K-region) ring opening在FSO 3 H / SO 2 ClF中通过低温质子化作用,由异构的单烷基化和二烷基化的苯并[ a ]蒽(BAs)生成了一系列新型的碳正离子化反应。C-7具有单烷基衍生物(5-甲基,6-甲基,7-甲基和7-乙基)以及D环甲基化类似物(9-甲基,10-甲基和11-甲基),或在所有情况下均观察到C-12质子化的碳正离子(作为唯一或主要的碳正离子)。12-甲基衍生物的质子化(9)得到C-7质子化的碳正离子(9H +)作为动能种类和本位-protonated碳阳离子(9AH +)作为热力学阳离子。与12-乙基衍生物(10),在箱式区域空间张力的浮雕大大有利于本位-protonation(10AH +)。具有3,9-二甲基(14),C-7质子化(14H +)(C-12质子化<10%)受到强烈青睐,在1,12-二甲基(15)的情况下,观察到的唯一物质是C-7质子化的碳正离子化(15H +)。对于7-甲
-
Coördination of Polycyclic Aromatic Hydrocarbons with Silver Ion; Correlation of Equilibrium Constants with Relative Carcinogenic Potencies<sup>1</sup>作者:Robert E. Kofahl、Howard J. LucasDOI:10.1021/ja01644a020日期:1954.8
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Ag: MVol.B5, 1.3.3.17, page 114 - 116作者:DOI:——日期:——
-
The Synthesis of Polynuclear Aromatic Hydrocarbons. I. Methyl-1,2-benzanthracenes<sup>1</sup>作者:Melvin S. Newman、Russell GaertnerDOI:10.1021/ja01157a072日期:1950.1
表征谱图
-
氢谱1HNMR
-
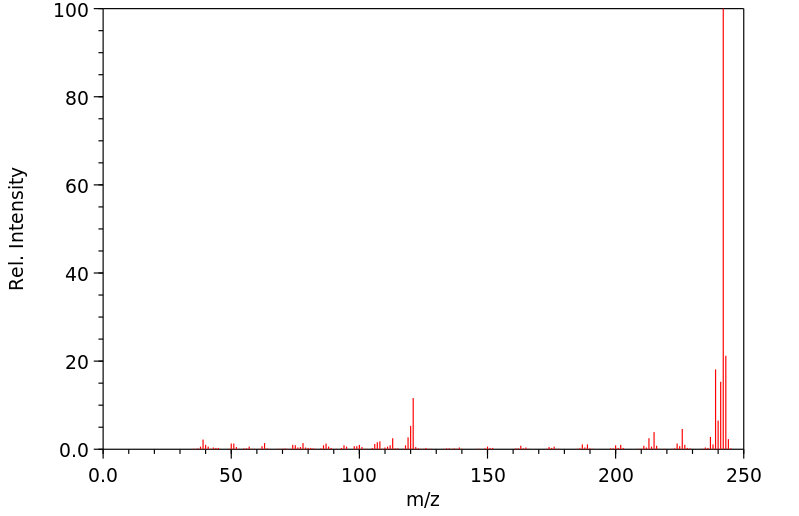
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩