trans-1,3,6-heptatriene | 44607-51-4
中文名称
——
中文别名
——
英文名称
trans-1,3,6-heptatriene
英文别名
hepta-1,3,6-triene;1,3,6-heptatriene;heptatriene-1,3,6;hepta-1,3t,6-triene;trans-1,3,6-Heptatrien;(E)-1.3.5-Heptatrien;(3E)-hepta-1,3,6-triene
CAS
44607-51-4
化学式
C7H10
mdl
——
分子量
94.1564
InChiKey
CXHZYOISZDAYCU-FNORWQNLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:55-56 °C(Press: 180 Torr)
-
密度:0.745 g/cm3
-
保留指数:728.8
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:trans-1,3,6-heptatriene 以 正戊烷 为溶剂, 反应 10.0h, 以11%的产率得到3-allylcyclobutene参考文献:名称:The 185-nm photolysis and pyrolysis of the spirocyclopropane-substituted azoalkanes of 2,3-diazatricyclo[4.3.0.04,9]non-2-ene and their denitrogenated hydrocarbon products, the tricyclo[3.2.0.02,7]heptanes摘要:The 185-nm photolysis and pyrolysis of the spirocyclopropane derivatives of the azoalkanes 2,3-diazatricyclo[4.3.0.0(4,9)]non-2-ene (1a), 4',5'-diazaspiro(cyclopropane-1,8'-tricyclo[4.3.0.0(3,7)]non-4'-ene) (1b), and 4'5'-diazadispiro(cyclopropane-1,2'-tricyclo[4.3.0.0(3,7)]non-4'-ene-8',1"-cyclopropane) (1c) and their denitrogenated hydrocarbon derivatives tricyclo[3.2.0.0(2,7)]heptane (2a), spiro(cyclopropane-1,3'-tricyclo[3.2.0.0(2,7)]heptane) (2b), and dispiro(cyclopropane-1,3'-tricyclo[3.2.0.0(2,7)]heptane-6'1"-cyclopropane) (2c) were investigated. It was shown that the 185-nm photochemical behavior of these substrates does not depend on the degree of spirocyclopropane substitution. As common products in the 185-nm photolysis of the azoalkanes 1a-c were obtained the tricycloalkanes 2a-c (major products), the norbornenes 3a-c, the vinylcyclopentenes 5a-c (minor products), and the exo-methylenecyclohexenes 6a-c (traces). In the case of the parent azoalkane 1a additionally bicyclo[3.2.0]hept-2-ene (4) and bicyclo[4.1.0]hept-2-ene (7a) were detected. The major products in the photolysis of the tricycloheptanes 2a-c were the vinylcyclopentenes 5a-c, but also the norbornenes 3a-c and the methylenecyclohexenes 6a-c were formed in considerable amounts. Although the norbornenes 3a-c and the bicyclo[3.2.0]heptene 4a are logical Wagner-Meerwein rearrangement products, attempts to trap the suspected radical-cationic and zwitterionic intermediates with CF3CH2OH failed. Efforts to generate the authentic radical-cationic species by means of photosensitized electron transfer (PET) by using sensitizers such as cyanoarenes, quinones, and pyrylium salts were unproductive. Vibrationally excited bicyclo[2.2.1]hepta-2,7-diyls, generated by the pyrolyses of 2a-c, are not precursors to the norbornenes 3a-c because, instead of such rearrangement products, cyclobutane cleavage of the bicyclo[2.1.0]pentane moiety takes place to afford the isomeric vinylcyclopentenes 5'a-c. Carbene intermediates, produced either from the 1,3-diyl-type species through fragmentation or from the photodenitrogenation of diazoalkanes, which are generated by retro-cleavage of n,pi* excited azoalkanes 1a-c, in turn obtained through internal conversion of higher excited states such as 1-pi,pi*, 1n,sigma*, and R(y), are proposed as the most likely precursors to either the vinylcyclopentenes 5a-c or methylenecyclohexenes 6a-c, respectively. In violation of Kasha's rule, photochemistry directly from the higher excited states of the azoalkanes 1a-c competes with internal conversion to the lowest excited state, namely the n,pi* state, as it was shown by the formation of norbornenes 3a-c.DOI:10.1021/jo00010a026
-
作为产物:描述:6,7-diazabicyclo[3.2.2]nona-2,6-diene 以7%的产率得到参考文献:名称:UYEHARA, TADAO;TAKAHASHI, MASAYUKI;KATO, TADAHIRO, TETRAHEDRON LETT., 1985, 26, N 9, 1241-1244摘要:DOI:
文献信息
-
Methyltriphenoxyphosphonium iodide (MTPI); induced dehydration and dehydrohalogenation in aprotic solvents作者:Charles W. Spangler、Douglas P. Kjell、Lori L. Wellander、Mary A. KinsellaDOI:10.1039/p19810002287日期:——Methyltriphenoxyphosphonium iodide (MTPI) is an effective dehydration and dehydrohalogenation reagent under mild conditions when 1,3-dimethylimidazolidin-2-one is used as an aprotic solvent in place of the more normally used hexamethylphosphoric triamide (HMPT). Since previously suggested mechanisms had proposed alcohol–HMPT interaction as an important mechanistic step, this result coupled with comparative
-
One‐pot Synthesis of 1,3‐Butadiene and 1,6‐Hexanediol Derivatives from Cyclopentadiene (CPD) via Tandem Olefin Metathesis Reactions作者:Gábor Turczel、Ervin Kovács、Eszter Csizmadia、Tibor Nagy、Imre Tóth、Robert TubaDOI:10.1002/cctc.201801088日期:2018.11.7A novel tandem reaction of cyclopentadiene leading to high value linear chemicals via ruthenium catalyzed ring opening cross metathesis (ROCM), followed by cross metathesis (CM) is reported. The ROCM of cyclopentadiene (CPD) with ethylene using commercially available 2nd gen. Grubbs metathesis catalysts (1‐G2) gives 1,3‐butadiene (BD) and 1,4‐pentadiene (2) (and 1,4‐cyclohexadiene (3)) with reasonable报道了一种新的环戊二烯串联反应,该反应通过钌催化的开环交叉复分解(ROCM),然后进行交叉复分解(CM),产生了高价值的线性化学品。环戊二烯(CPD)与乙烯的ROCM使用可商购的第二代产品。Grubbs复分解催化剂(1-G2)产生1,3-丁二烯(BD)和1,4-戊二烯(2)(和1,4-环己二烯(3)),且产率合理(高达24%(BD)和67甲苯溶液中1–5 mol%的催化剂负载量(5 V%CPD,在73%CPD转化率下为%(2 + 3)),10 bar,RT)在平衡反应中。CPD与顺丁烯二醇二乙酸酯(4)的ROCM含量为1.00 ‐ 0.05 mol%的第三代。Grubbs(1-G3)或第二代 Hoveyda-Grubbs(1-HG 2)催化剂的负载量得到六乙酸2,4-二烯-1,6-二乙酸二乙酯(5),它是1,6-己二醇(聚氨酯,聚酯和多元醇合成的中间体)的前体以及高收率的二庚基2,5-二烯-1
-
Bloch, R.; Abecassis, J.; Hassan, D., Canadian Journal of Chemistry, 1984, vol. 62, p. 2019 - 2024作者:Bloch, R.、Abecassis, J.、Hassan, D.DOI:——日期:——
-
Organic photochemistry with 6.7-eV photons: the photochemistry of bicyclo[4.1.0]hept-2-ene (2-norcarene)作者:William J. Leigh、R. SrinivasanDOI:10.1021/ja00341a034日期:1983.2
-
Green, Iain G.; Walton, John C., Journal of the Chemical Society. Perkin transactions II, 1984, # 7, p. 1253 - 1258作者:Green, Iain G.、Walton, John C.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
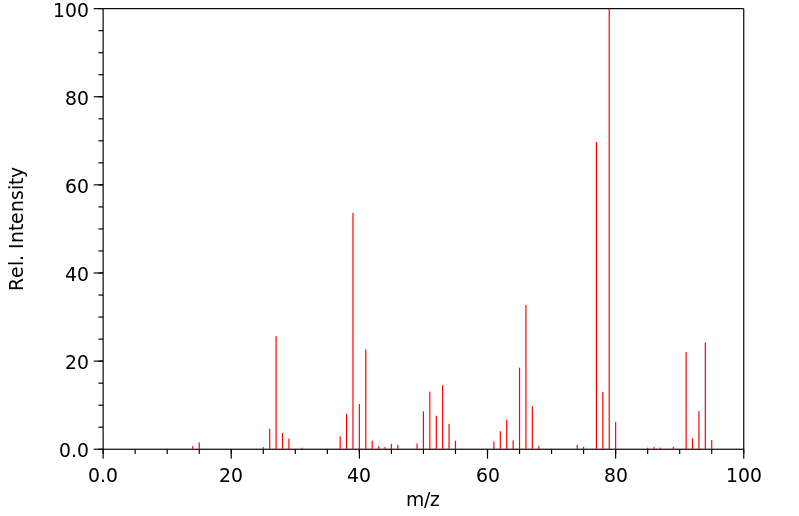
质谱MS
-
碳谱13CNMR
-
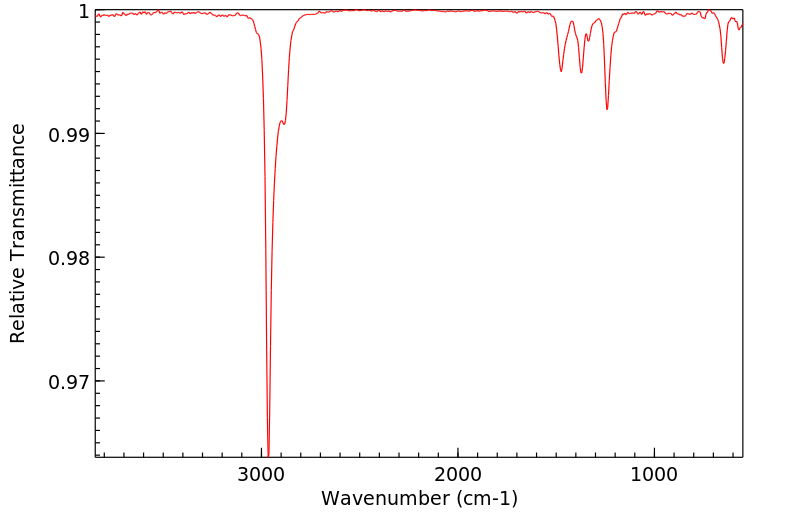
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-