四乙基丙烯-1,3-二膦酸酯 | 22401-25-8
物质功能分类
中文名称
四乙基丙烯-1,3-二膦酸酯
中文别名
1,3-丙二膦酸四乙基酯
英文名称
1,3-bis(diethoxyphosphinyl)propane
英文别名
tetraethyl propane-1,3-diylbis(phosphonate);tetraethylpropane 1,3-diylbis(phosphonate);tetraethyltrimethylenediphosphonate;1,3-bis(diethoxyphosphoryl)propane;propanediyl-bis-phosphonic acid tetraethyl ester;Propandiyl-bis-phosphonsaeure-tetraaethylester;Phosphonic acid, trimethylenedi-, tetraethyl ester
CAS
22401-25-8
化学式
C11H26O6P2
mdl
MFCD00048543
分子量
316.271
InChiKey
LJQJJGQRJQSYQN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:206-227 °C (5 mmHg)
-
密度:1.114±0.06 g/cm3 (20 ºC 760 Torr)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:19
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:71.1
-
氢给体数:0
-
氢受体数:6
安全信息
-
海关编码:2915900090
-
安全说明:S24/25
-
储存条件:存储于阴凉干燥处。
SDS
| Name: | Tetraethylpropylene-1 3-diphosphonate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 22401-25-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 22401-25-8 | Tetraethylpropylene-1,3-diphosphonate | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 22401-25-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Viscous liquid
Color: Clear
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 206 - 227 deg C @ 5
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C11H26O6P2
Molecular Weight: 316.126
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of phosphorus, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 22401-25-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tetraethylpropylene-1,3-diphosphonate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 22401-25-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 22401-25-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 22401-25-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二乙基(3-溴丙基)膦酸酯 Diethyl 3-bromopropylphosphonate 1186-10-3 C7H16BrO3P 259.08
反应信息
-
作为反应物:描述:参考文献:名称:[EN] NOVEL MANGANESE COMPRISING NANOSTRUCTURES
[FR] NOUVELLES NANOSTRUCTURES COMPORTANT DU MANGANÈSE摘要:本文披露了包含至少五个伽那双膦酸酯基团的聚合物框架的纳米结构,其中这些伽那双膦酸酯基团独立地被结合为-R3R4C(P=O(OR1)(OR2))2,其中R1和R2分别从阴离子、H、烷基和芳基组成的组中独立选择,且其中R3和R4中至少有一个是连接到聚合物框架的基团,但有一个限制条件,即当R3和R4中仅有一个是这样的连接基团时,另一个R3和R4要么是能够连接到聚合物框架的基团,要么是这种基团的残基,或者从H、OH、OR5和R5组成的组中选择,其中R5是较低的烷基。聚合物框架可能包括锰离子。还披露了制备含锰的纳米结构的方法,包含这种含锰纳米结构的组合物以及使用这种含锰纳米结构,例如作为MRI对比剂。公开号:WO2013041623A1 -
作为产物:描述:参考文献:名称:Die Pyro- und die Unterphosphors�ure im Vergleich mit organischen Diphosphons�uren摘要:DOI:10.1007/bf00899234
文献信息
-
Direct Conversion of Phosphonates to Phosphine Oxides: An Improved Synthetic Route to Phosphines Including the First Synthesis of Methyl JohnPhos作者:Alexander J. Kendall、Chase A. Salazar、Patrick F. Martino、David R. TylerDOI:10.1021/om500854u日期:2014.11.10reaction intermediate. A diverse array of phosphonates was converted to phosphine oxides using a variety of Grignard reagents for direct carbon–phosphorus functionalization. This new methodology especially simplifies the synthesis of dimethylphosphino (RPMe2)-type phosphines by using air-, water-, and silica-stable intermediates. To highlight this reaction, a new Buchwald-type ligand ([1,1′-biphenyl]-使用化学计量的烷基或芳基格氏试剂和三氟甲烷磺酸钠(NaOTf),可以可靠地实现由膦酸酯合成叔膦氧化物,并以优异的收率获得良好的收率。在没有NaOTf添加剂的情况下,镁和磷物种的共价配位低聚物占主导地位,产生了非常低的氧化膦收率,但是膦酸酯原料的转化率很高。机理研究表明,五配位的磷物质(不是次膦酸盐)是反应的中间体。使用多种用于直接碳-磷官能化的格氏试剂,将各种各样的膦酸酯转化为氧化膦。这种新方法特别简化了二甲基膦基(RPMe 2)型膦通过使用对空气,水和二氧化硅稳定的中间体。为了突出该反应,以优异的产率合成了新的布赫瓦尔德型配体([1,1'-联苯] -2-基二甲基膦或甲基JohnPhos)和经典的双齿膦双(二苯基膦基)丙烷(dppp)。
-
Nanostructures comprising manganese申请人:Spago Imaging AB公开号:EP2572736A1公开(公告)日:2013-03-27Disclosed herein are nanostructures comprising manganese ions incorporated in a polymeric framework comprising at least five geminal bisphosphonate groups, wherein the geminal bisphosphonate groups independently of each other are incorporated as -R3R4C(P=O(OR2)(OR2))2, wherein R1 and R2 are independently selected from the group consisting of a negative charge, H, alkyl and aryl, and wherein at least one of R3 and R4 is a group connected to the polymeric framework with the proviso that when only one of R3 and R4 is such a connected group, the other of R3 and R4 is either a group being able to connect to the polymeric framework, or the residue of such a group, or selected from the group consisting of H, OH, OR5 and R5, wherein R5 is a lower alkyl. Disclosed are also methods for producing such nanostructures, compositions comprising such nanostructures and use of such nanostructures, i.a. as MRI contrasting agents.
-
Turn-on fluorescence sensor for mono- and di-phosphonic acid derivatives using anthracene-based diamidine and its detection of amidinium-phosphonate and amidinium formation作者:Takahiro Kusukawa、Hitoshi Nagano、Keita Nakaguchi、Shota Takeshita、Yuya HarumotoDOI:10.1016/j.tet.2017.12.011日期:2018.1di-phosphonic acid and mono-phosphonic acid derivatives using the anthracene-based diamidine 1 has been investigated. The diamidine 1 forms 1:1 and 1:2 complexes with the di-phosphonic acid and mono-phosphonic acid derivatives, respectively, and showed a blue fluorescence (λem = 432–442 nm) in a DMSO solution. The formation of amidinium-phosphonate (complex formation) and dissociated amidinum (λem = 468 nm as a
-
Organische Phosphorverbindungen XVII. Darstellung von Alkylen-bis-phosphonsäurechloriden und Alkylen-bis-thiophosphonsäurechloriden und deren Reaktion mit G<scp>RIGNARD</scp>-Verbindungen作者:Ludwig MaierDOI:10.1002/hlca.19650480114日期:——The synthesis and some physical properties of alkylene bis-(phosphonic dichlorides), Cl2P(O)(CH2)n(O)PCl2, and of alkylene bis-(phosphonothioic dichlorides), Cl2P(S)(CH2)n(S)PCl2, a new class of compounds, are reported. The reactions of alkylene bis-(phosphonothioic dichlorides) with GRIGNARD reagents and of CH3P(S)Br2 with di-GRIGNARD reagents are also described. Some physical properties of several
-
Synthesen, chemische Reaktionen und NMR-spektroskopische Untersuchungen substituierter Phosphonopyruvate
表征谱图
-
氢谱1HNMR
-
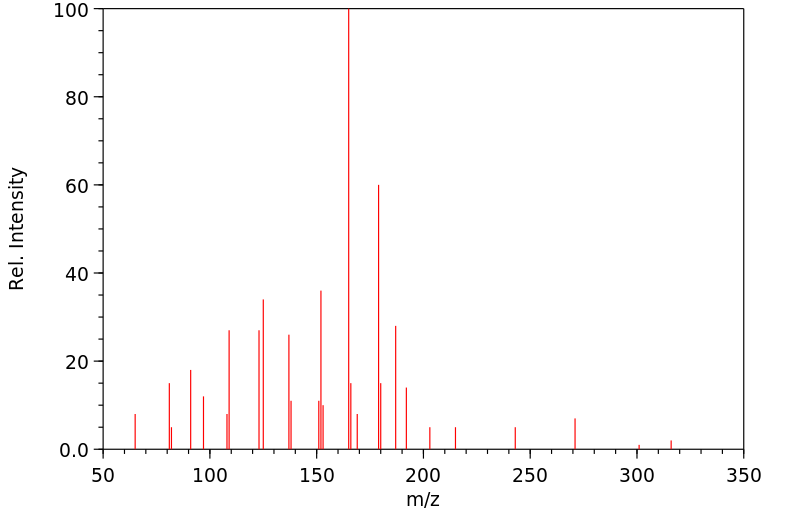
质谱MS
-
碳谱13CNMR
-
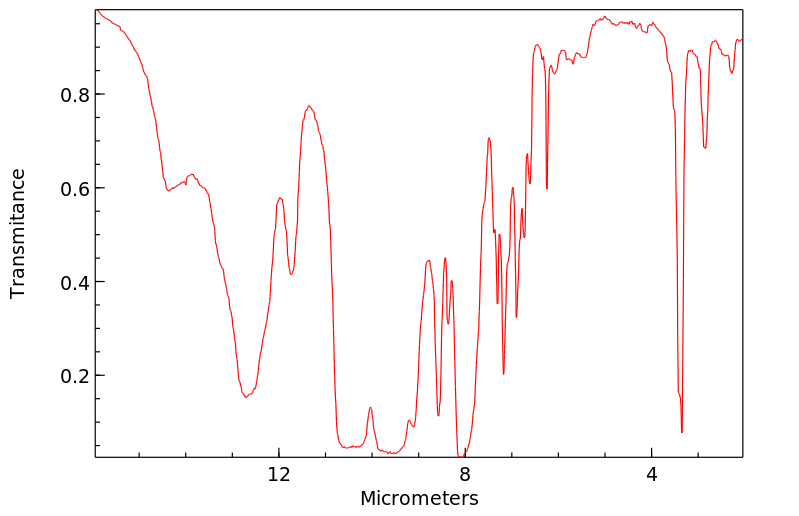
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1-氨基丁基)磷酸
顺丙烯基磷酸
除草剂BUMINAFOS
阿仑膦酸
阻燃剂 FRC-1
铵甲基膦酸盐
钠甲基乙酰基膦酸酯
钆1,5,9-三氮杂环十二烷-N,N',N''-三(亚甲基膦酸)
钆-1,4,7-三氮杂环壬烷-N,N',N''-三(亚甲基膦酸)
重氮甲基膦酸二乙酯
辛基膦酸二丁酯
辛基膦酸
辛基-膦酸二钾盐
辛-1-烯-2-基膦酸
试剂12-Azidododecylphosphonicacid
英卡膦酸
苯胺,4-乙烯基-2-(1-甲基乙基)-
苯甲基膦酸二甲酯
苯基膦酸二甲酯
苯基膦酸二仲丁酯
苯基膦酸二乙酯
苯基膦酸二乙酯
苯基磷酸二辛酯
苯基二异辛基亚磷酸酯
苯基(1H-1,2,4-三唑-1-基)甲基膦酸二乙酯
Tetrapotassium (((2-hydroxyethyl)imino)bis(methylene))bisphosphonate
苄基膦酸苄基乙酯
苄基亚甲基二膦酸
膦酸,[(2-乙基己基)亚氨基二(亚甲基)]二,triammonium盐(9CI)
膦酸叔丁酯乙酯
膦酸单十八烷基酯钾盐
膦酸二辛酯
膦酸二(二十一烷基)酯
膦酸,辛基-,单乙基酯
膦酸,甲基-,单(2-乙基己基)酯
膦酸,甲基-,二(苯基甲基)酯
膦酸,甲基-,2-甲氧基乙基1-甲基乙基酯
膦酸,丁基乙基酯
膦酸,[苯基[(苯基甲基)氨基]甲基]-,二甲基酯
膦酸,[[羟基(苯基甲基)氨基]苯基甲基]-,二(苯基甲基)酯
膦酸,[2-(环丙基氨基)-2-羰基乙基]-,二乙基酯
膦酸,[2-(二甲基亚肼基)丙基]-,二乙基酯,(E)-
膦酸,[1-甲基-2-(苯亚氨基)乙烯基]-,二乙基酯
膦酸,[1-(乙酰基氨基)-1-甲基乙基]-(9CI)
膦酸,[(环己基氨基)苯基甲基]-,二乙基酯
膦酸,[(二乙氧基硫膦基)(二甲氨基)甲基]-
膦酸,[(2S)-2-氨基-2-苯基乙基]-,二乙基酯
膦酸,[(1Z)-2-氨基-2-(2-噻嗯基)乙烯基]-,二乙基酯
膦酸,P-[(二乙胺基)羰基]-,二乙基酯
膦酸,(氨基二环丙基甲基)-